Home » Research
Research
Research projects
Prevention of Hereditary Breast Cancer through Personalized Optimization of Se, Zn, Fe Levels in the Body Using Dietary Supplements
Agreement No. INNOMED/I/16/NCBR/2014
Project Duration as per the Agreement: 01.10.2014-30.09.2019. The project co-financed by the European Union under the European Regional Development Fund
The project partner is the West Pomeranian University of Technology in Szczecin
Characteristics of the studied groups
The study groups, whose analyses form the basis for the results described below, were created from individuals registered in the Hereditary Cancer Center in Szczecin between 2009 and 2020. Each patient provided informed consent for the storage and use of biological material for scientific purposes. Blood and serum samples were collected between 8 AM and 2 PM, and patients fasted for at least 4 hours before collection. For the majority of patients, a sample was collected only once, but in some cases, multiple samples were taken during subsequent visits. Biological material was stored at -80°C until the element concentrations were determined. Each participant in the study completed a health and lifestyle questionnaire.
A total of 2962 healthy women were included in the prospective cohort. During 42 months of follow-up, 148 women were diagnosed with cancer.
The characteristics of the group are presented in the table.
| Cases (n=148) | Healthy (n=2814) | |
| Average age (range) | 56,46 (35-82) | 53 (33-84) |
Smoking current former never | 40 (27,03%) 34 (22,97%) 74 (50 %) | 605 (21,50%) 750 (26,65%) 1459 (51,84%) |
Hormone replacement therapy / oral contraceptives no yes missing data | 93 (62,84%) 54 (36,49%) 1 (0,67%) | 1467 (52,13%) 1316 (46,77%) 31 (1,1%) |
Adnexektomia no yes missing data | 135 (91,22%) 9 (6,08%) 4 (2,7%) | 2631 (93,50%) 175 (6,22%) 8 (0,28%) |
Cancers (location) breast uterus colon melanoma thyroid ovary cervix lymphoma bladder lung kidney stomach leukemia myeloma glioblastoma pancreas |
76 (51,4%) 12 (8,1%) 10 (6,8%) 7 (4,7%) 7 (7,73%) 6 (4,05%) 5 (3,38%) 5 (3,38%) 4 (2,7%) 4 (2,7%) 3 (2,03%) 3 (2,03%) 2 (1,35%) 2 (1,35%) 1 (0,68%) 1 (0,68%) |
– – – – – – – – – – – – – – – – |
| Cases (n=107) | Healthy (n=1217) | |
| Average age (range) | 44,25 (26-66) | 40,5 (25-69) |
| Smoking
current former never missing data |
24 (22,43%) 27 (25,23%) 55 (51,4%) 1 (0,93%) |
254 (20,87%) 249 (20,46%) 699 (57,44%) 15 (1,23%) |
| Hormone replacement therapy / oral contraceptives
no yes missing data |
46 (43%) 60 (56,07%) 1 (0,93%) |
603 (49,55%) 592 (48,64%) 22 (1,81%) |
| Adnexektomia
no yes missing data |
52 (48,6%) 55 (51,4%) 0 |
688 (56,53%) 510 (41,92%) 19 (1,56%) |
| Cancers (location)
breast ovary cervix peritoneum stomach colon leukemia pancreas skin larynx thyroid bladder |
80 (74,8%) 15 (14%) 3 (2,8%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) |
– – – – – – – – – – – – |
| Cases (n=144) | Healthy (n=2812) | |
| Average age (range) | 60,5 (36-76) | 52 (31-87) |
| Smoking
current former never |
42 (29,17%) 55 (38,19%) 47 (32,64 %) |
829 (29,48%) 975 (34,67%) 1008 (35,85%) |
| Cancers (location)
prostate skin kidney colon bladder blood lung liver thyroid pancreas stomach breast esophagus pituitary gland salivary glands testis |
58 (40,28%) 16 (11,11%) 13 (9,03%) 13 (9,03%) 12 (8,33%) 9 (6,25%) 6 (4,17%) 4 (2,78%) 4 (2,78%) 2 (1,39%) 2 (1,39%) 1 (0,69%) 1 (0,69%) 1 (0,69%) 1 (0,69%) 1 (0,69%) |
– – – – – – – – – – – – – – – – |
| Alive (n=417) | Deaths (n=121) | |
| Average age (range) | 55,87 (25-85) | 61,07 (28-91) |
| Smoking
current former never missing data |
89 (21,3%) 112 (26,9%) 206 (49,4%) 10 (2,4%) |
26 (21,5%) 27 (22,3%) 65 (53,7%) 3 (2,5%) |
| Estrogen receptor
positive (ER+) negative (ER-) missing data |
287 (68,8%) 114 (27,3%) 16 (3,8%) |
79 (65,3%) 32 (26,4%) 10 (8,26%) |
| BRCA1 mutation | 50 (12%) | 11 (9,1%) |
| Chemotherapy
yes no missing data |
211 (50,6%) 174 (41,7%) 32 (7,7%) |
65 (53,7%) 36 (29,8%) 20 (16,5%) |
| Radiotherapy
yes no missing data |
244 (58,5%) 122 (29,3%) 51 (12,2%) |
56 (46,3%) 35 (28,9%) 30 (24,8%) |
| Tamoxifen
yes no missing data |
282 (67,6%) 122 (29,3%) 13 (3,1%) |
79 (65,3%) 33 (27,3%) 9 (7,4%) |
| Type of operation
mastectomy lumpectomy missing data |
261 (62,6%) 131 (31,4%) 25 (6%) |
82 (67,8%) 17 (14%) 22 (18,2%) |
The study included 357 patients with diagnosed and histopathologically confirmed prostate cancer. Blood was collected between 2009 and 2015 at the time of diagnosis of prostate cancer before treatment. The average follow-up period for patients was 5 years.
| Gleason scale | Alive (n=293) | Deaths (n=102) |
6 7 8 9 10 missing data | 85 (29%) 164 (56%) 20 (6,8%) 17 (5,8%) 2 (0,7%) 5 (1,7%) | 39 (38,2%) 35 (34,3%) 9 (8,8%) 11 (10,8%) 7 (6,9%) 2 (1,96%) |
| Clinical stage 1-2 3 4 missing data | 7 (2,7%) 232 (79,2%) 51 (17,4%) 2 (0,7%) | 4 (3,9%) 45 (44,1%) 53 (52%) – |
| Radiotherapy yes no | 84 (28,7%) 209 (71,3%) | 24 (23,5%) 78 (76,5%) |
| Chemotherapy yes no | 3 (1%) 290 (99%) | 4 (3,9%) 98 (96,1%) |
| Hormonetherapy yes no | 59 (20,1%) 234 (79,9%) | 40 (39,2%) 62 (60,8%) |
| Prostatectomy yes no | 233 (79,5%) 60 (20,5%) | 33 (32,4%) 69 (67,6%) |
| Orchidectomy yes no | 8 (2,7%) 285 (97,3%) | 11 (10,8%) 91 (89,2%) |
| n=315 | % | |
| Average age of cancer diagnosis (range) | 61,1 (41-86) | |
| Sex
women men |
49 266 |
15,6 84,4 |
| Clinical stage
1 2 3 4 |
72 42 70 131 |
22,8 13,3 22,2 41,6 |
| Pack-years (average, range) | 37,11 (0-150) | |
| Treatment
radiotherapy chemotherapy |
142 32 |
45,1 10,1 |
| % | ||
| Sex
Men Women |
196 106 |
64,9 35,1 |
| Average age (range) | 64,2 (43-86) | – |
| Pack-years (range) | 33,2 (0-232,8) | – |
| Smoking
yes no |
283 19 |
93,7 6,3 |
| Clinical stage
1 2 3 4 |
129 75 79 19 |
42,7 24,8 26,2 6,3 |
| Radiotherapy
yes no |
78 224 |
25,8 74,2 |
| Chemotherapy
yes no |
105 197 |
34,8 65,2 |
| Histological type
Adenocarcinoma Squamous cell carcinoma Large cell cancer Large and Small Cell Mixed Carcinoma Small Cell Carcinoma Other |
136 124 21 5 3 13 |
45 41,1 7 1,7 1 4,3 |
| New cancer diagnosis | |
| Year of birth (range) | 1930-1976 |
| Average age at the blood donation (range) | 63,4 (35-84) |
| Sex
men women |
59 41 |
| First-Degree Relatives
with pancreatic cancer with other cancer |
4 43 |
| Smoking
yes no |
27 73 |
| Pack-years (range) | 28,99 (2-50) |
| Alive (n=344) | Deaths (n=31) | |
| Average age (range) | 54,6 (21-90) | 64,3 (38-86) |
| Sex
women men |
218 (63%) 126 (37%) |
14 (45%) 17 (55%) |
| Clinical stage
2 3 4-5 |
70 (20%) 145 (42%) 129 (38%) |
1 (3,2%) 12 (39%) 18 (58%) |
Research results
In the body, selenium acts through proteins into which it is incorporated in the form of selenocysteine. As a component of selenoproteins, selenium plays an enzymatic and structural role[1]. Some of the most important functions of selenoproteins include participation in the production of thyroid hormones, stimulation of the immune system, and protection against oxidative stress.[2]
Both deficiency and excess of this element may harm the body. This leads to, among others: heart disorders, heart and liver degeneration, increased risk of hypertensive disease, reduced immune system efficiency, thyroid dysfunction, impaired bone mineralization and proper tooth formation, and increased risk of cancer. [3–7]
Evaluation of the cancer risk in women - BRCA1 non-carriers
Women over 60 years old and non-smokers have a significantly 4.5-fold reduced risk if their blood concentration is in the range of 94-104 µg/l. However, women over 60 years of age who currently smoke or have smoked in the past have a significantly 3.5-fold reduced risk of cancer if their blood selenium concentration is > 110 µg/l.
Incidence of cancer in non-smoking women over 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 94-104 | 3 | 114 | Ref. | Ref. | Ref. |
| II | <94 | 11 | 93 | 4,5 | 1,2-16,6 | 0,02* |
*statistically significant result (p<0.05) P.438038
Incidence of cancer in smoking women over 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | >110 | 4 | 97 | Ref. | Ref. | Ref. |
| II | <110 | 25 | 171 | 3,5 | 1,2-10,5 | 0,02* |
*statistically significant result (p<0.05) P.438038
Evaluation of the cancer risk in women - BRCA1 carriers
Women under 50 years of age with blood selenium concentrations in the range of 70-80 µg/l tend to have a nearly 5-fold reduced risk of cancer compared to women with selenium concentrations outside the presented range.
Cancer incidence depending on blood selenium concentration among women under 50 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 70-80 | 1 | 56 | Ref. | Ref. | Ref. |
| II | <70 & >80 | 75 | 904 | 4,6 | 0,6-34,1 | 0,1* |
*result not statistically significant (p >0.05) P.435603
Women over 50 years of age with blood selenium concentrations in the range of 95-120 µg/l have a more than twofold reduced risk of cancer compared to women with selenium concentrations outside the presented range.
Cancer incidence depending on blood selenium concentration among women over 50 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 95-120 | 13 | 156 | Ref. | Ref. | Ref. |
| II | <95 & >120 | 20 | 102 | 2,3 | 1,1-4,9 | 0,03* |
*statistically significant result (p <0.05) P.435603
Evaluation of the cancer risk in men
Men who do not smoke cigarettes and have selenium concentrations in the blood range of 100-110 µg/l have a nearly 4-fold reduced risk of cancer compared to men with selenium concentrations outside this range.
Cancer incidence depending on blood selenium concentration among non-smoking men (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 100-110 | 5 | 260 | Ref. | Ref. | Ref. |
| II | <95 | 20 | 291 | 3,6 | 1,3-9,7 | 0,008* |
*statistically significant result (p < 0.05) P.437898
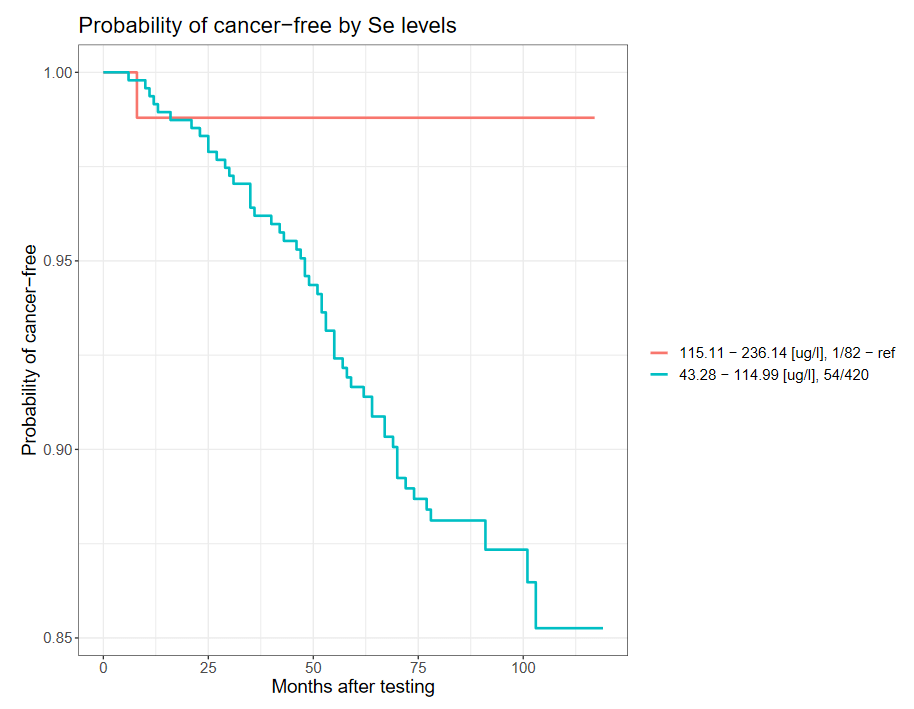
However, men over 60 years of age and those who have smoked cigarettes in the past or currently have a nearly 11-fold reduced risk of developing cancer if the selenium concentration in the blood is >115 µg/l, compared to men with the selenium concentration in the blood <115 µg/ l.
Cancer incidence depends on blood selenium concentration among smoking men over 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <115 | 1 | 83 | Ref. | Ref. | Ref. |
| II | ≥115 | 56 | 427 | 10,9 | 1,5-79,8 | 0,0013* |
*statistically significant result (p < 0.05) P.437898
The Kaplan-Meier curve for the above correlation is shown below.
-
-
Survival of patients with breast cancer
Women with breast cancer have a significantly greater chance of 10-year survival if their serum selenium concentration is >94.7 µg/l. Women with serum selenium concentration < 70 µg/l have a more than 25-fold increased risk of death compared to the subgroup with serum selenium concentration 95-102.5 µg/l.
Incidence of death depending on serum selenium concentration in women with breast cancer within 10 years of diagnosis (quarters).
| Quartiles | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | 52,1-76,7 | 89 | 46 | 2,35 | 1,12-4,55 | 0,01* |
| II | 76,8-85,1 | 105 | 29 | 1,52 | 0,76-3,02 | 0,23 |
| III | 85,2-94,6 | 105 | 29 | 1,95 | 1,01-3,76 | 0,047* |
| IV | 94,7-171,5 | 118 | 17 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The incidence of death depends on serum selenium concentration in women with breast cancer within 10 years of diagnosis (ranges).
| Quartiles | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | < 70 | 32 | 28 | Ref. | Ref. | Ref. |
| II | 95-102,5 | 62 | 2 | 27,1 | 6,7-121,2 | <0,0001* |
*statistically significant result (p<0.05)
The above results are part of the publication by Szwiec M et al., Serum Selenium Level Predicts 10-Years Survival after Breast Cancer; Nutrients 2021, 13(3), 953. [8]
Survival of patients with prostate cancer
Men with prostate cancer and serum selenium concentration in the range of 85-105 µg/l have a significantly reduced risk of death by over 8 times compared to the subgroup with serum selenium concentration below 70 µg/l.
The incidence of death depends on serum selenium concentration in men with prostate cancer within 5 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | ≤70 | 61 | 33 | 8,6 | 3,4-21,7 | <0,0001* |
| II | 85-105 | 95 | 6 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
Survival of patients with lung cancer
It has been observed that people whose serum selenium concentration is >67.4 µg/l have a greater chance of longer survival after a lung cancer diagnosis than people with a low serum selenium concentration (<55.1 µg/l).
Frequency of deaths depending on serum selenium concentration in patients with stage I lung cancer within 3 years of diagnosis (tertiles).
| Tertiles | Range [µg/l] | total | OR | 95%CI | p |
| I | 33,46-57,91 | 43 | 2,73 | 1,21-6,11 | 0,01* |
| II | 57,92-68,86 | 42 | 1,88 | 0,83-4,28 | 0,13 |
| III | 69,29-108,27 | 44 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The above results are part of the publication by Pietrzak S et al., Influence of the selenium level on overall survival in lung cancer, Journal of Trace Elements in Medicine and Biology 2019, 56:46-51.[9]
Survival of patients with pancreatic cancer
Patients diagnosed with pancreatic cancer have a 3-fold greater chance of 6-month survival if their serum selenium concentration is ≥ 63.67 µg/l compared to patients with selenium concentration below this value.
The incidence of death depends on serum selenium concentration in patients with pancreatic cancer within 6 months of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | >< 63,67 | 24 | 33 | 0,4 | 0,1-0,97 | 0,03* |
| II | ≥ 63,67 | 22 | 11 | Ref. | Ref. | Ref. |
The above results are part of the publication by Lener M et al., Serum concentration of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer, Cancer Res Treat., 2016, 48(3):1056-1064.[10]
Survival of patients with melanoma
Patients diagnosed with malignant melanoma have a significantly greater chance of 10-year survival if their serum selenium concentration is higher than 96 µg/l. Patients with serum selenium concentration 96 µg/l.
The incidence of death depends on serum selenium concentration in patients with malignant melanoma within 10 years of diagnosis (quarters).
| Quartiles | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | 56,7-76,2 | 78 | 16 | 5,83 | 1,32-25,8 | 0,02* |
| II | 76,4-85,01 | 86 | 7 | 3,37 | 0,7-16,3 | 0,13 |
| III | 85,15-96,06 | 88 | 6 | 3,34 | 0,67-16,7 | 0,14 |
| IV | 96,15-168 | 92 | 2 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The above results are the subject of the publication by Rogoża-Janiszewska E. et al., Serum selenium level and the 10-year survival after melanoma, Biomedicines, 2021.[11]
Arsenic and its compounds are among the most recognized toxins. According to the classification of the International Agency for Research on Cancer (IARC), arsenic and its compounds have been categorized as Group 1[12] carcinogens, meaning they are definite human carcinogens. The clinical manifestations resulting from inhalation or ingestion of arsenic compounds vary widely. Depending on the concentration, duration of exposure, and route of absorption, the effects of arsenic interaction with tissues range from relatively benign, such as hypopigmentation, to life-threatening tumors (WHO). Based on existing literature, it can be concluded that not only high but also slightly elevated arsenic concentrations may be linked to cancers, especially in women.
Evaluation of the cancer risk in women - BRCA1 non-carriers
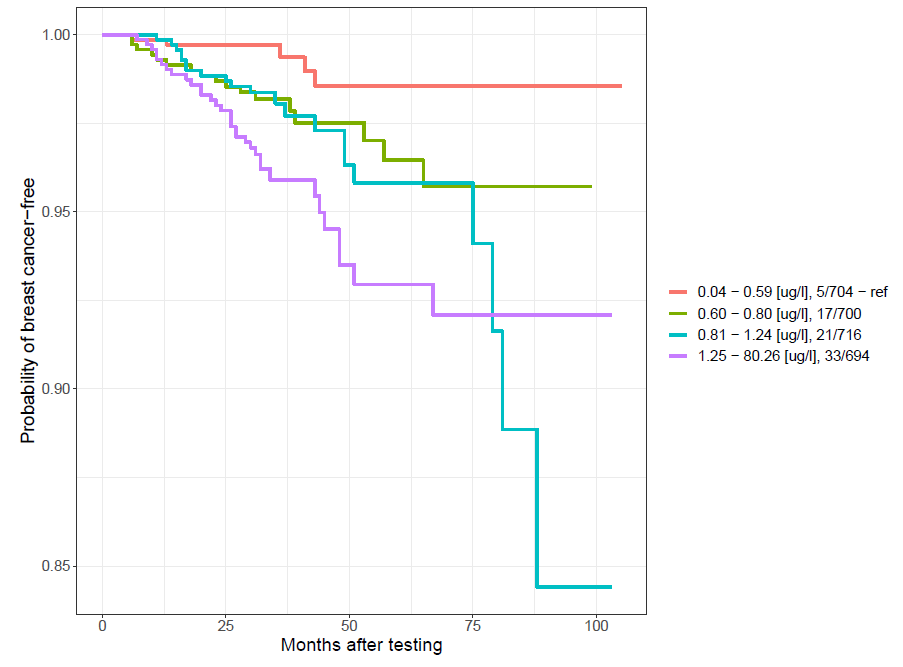
Women with an arsenic concentration in blood below 0.6 µg/l have a significant, almost 5-fold reduced risk of developing cancers, especially breast cancer, compared to women with an arsenic concentration above 0.6 µg/l (OR=4.7; p=0 .0004; 95%CI:1.9-11.7).
Incidence of breast cancer depending on arsenic concentration in blood (selected ranges)
| Group | Range | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <0,6 | 5 | 735 | Ref. | Ref. | Ref. |
| II | 0,6-0,81 | 18 | 723 | 3,7 | 1,4-10 | 0,01* |
| III | 0,82-1,25 | 21 | 719 | 4,3 | 1,6-11-5 | 0,002* |
| IV | >1,25 | 32 | 709 | 6,6 | 2,6-17,1 | <0,0001* |
| Selected ranges | ||||||
| I | <0,6 | 5 | 735 | Ref. | Ref. | Ref. |
| II | ≥0,6 | 71 | 2222 | 4,7 | 1,9-11,7 | 0,0004* |
*statistically significant result (p<0.05) P.425602
The Kaplan-Meier curve for the above correlation is shown below.
-
The above results are the subject of the publication Marciniak W. et al., Blood arsenic levels and the risk of familial breast cancer in Poland, Int J Cancer, 2020, 146 (10): 2721-2727.[13]
Evaluation of the cancer risk in women - BRCA1 carriers
Women with an arsenic concentration in blood below 0.85 µg/l have a significantly reduced risk of developing cancer by approximately two times compared to women with an arsenic concentration above 0.85 µg/l (OR=2.55; p=0.0006; 95% CI:1.47-4.43).
The incidence of cancer depends on the concentration of arsenic in the blood.
| Group | Range | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <0,85 | 18 | 513 | Ref. | Ref. | Ref. |
| II | >0,85 | 49 | 548 | 2,55 | 1,47-4,43 | 0,0006* |
*statistically significant result (p<0.05)
The above results are part of the publication by Marciniak W. et al., Blood Arsenic Levels as a Marker of Breast Cancer Risk among BRCA1 Carriers, Cancers 2021, 13(13), 3345. [14]
Evaluation of the cancer risk in men
Men whose blood arsenic concentration is between 0.7 and 1.14 µg/l have a nearly 5-fold reduced risk of cancer.
Cancer incidence in men depending on arsenic concentration in blood (selected ranges)
| Group | Range [µg/l] | .New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 0,7-1,14 | 28 | 788 | Ref. | Ref. | Ref. |
| II | <0,7 & > 1,14 | 116 | 2003 | 4,8 | 1,1-2,5 | 0,03* |
*statistically significant result (p<0.05) P.437896
Survival of patients with prostate cancer
Men with serum arsenic concentration in the range of 0.7-1.0 µg/l have a significantly reduced risk of death by more than 3 times compared to the subgroup with serum arsenic concentration < 0.7 µg/l.
The incidence of death depends on serum arsenic concentration in men with prostate cancer within 5 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Deaths | Alive | OR | 95%CI | p |
| I | <0,7 | 22 | 50 | 3,18 | 1,63-6,22 | 0,0009* |
| II | 0,7-1,0 | 22 | 159 | Ref. | Ref. | Ref. |
| III | >1,0 | 21 | 83 | 1,83 | 0,95-3,52 | 0,085 |
*statistically significant result (p<0.05)
According to the classification of the International Agency for Research on Cancer (IARC), cadmium and its compounds have been categorized as Group 1 [12] human carcinogens. The adverse effects of cadmium and its compounds can lead to kidney diseases, cardiovascular diseases, hypertension, anemia, liver damage, disorders of the reproductive organs, immune system disorders, deficiencies of iron, copper, and zinc, as well as the development of cancer. Numerous studies describe increased levels of cadmium in the biological material of individuals who have developed malignant tumors of the prostate, kidney, bladder, pancreas, and breast.
The literature mentions three main sources of cadmium: diet, smoking, and occupational exposure[25]. The concentration of cadmium in food products is strongly dependent on the content of this element in the environment - air, soil, and water[25]. The concentration of cadmium in the blood is strongly correlated with smoking tobacco products. In non-smokers, Cd concentration is lower compared to smokers[26]. The occupational group most exposed to cadmium are workers in the zinc, steel, and copper industries, as well as in the production of nickel-cadmium batteries, solar cells, and jewelry[27].
Evaluation of the cancer risk in women - BRCA1 non-carriers
The table below shows the distribution of subjects in our chosen range. The risk of cancer is reduced more than 8 times among women whose concentration is in the range of 0.28-0.33 µg/l.
Cancer incidence depending on blood cadmium concentration in non-smoking women over 50 years of age (selected range)
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 0,28-0,33 | 1 | 126 | Ref. | Ref. | Ref. |
| II | <0,28 & >0,33 | 44 | 665 | 8,34 | 1,1-61,1 | 0,009* |
*statistically significant result (p<0.05) P.437608
Evaluation of the cancer risk in women - BRCA1 carriers
In women under 51 years of age with blood cadmium concentration ≤0.32 µg/l, the risk is reduced by more than 8 times compared to women with higher cadmium levels.
Cancer incidence depending on blood cadmium concentration in women under 51 years of age (selected range)
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | ≤0,32 | 1 | 92 | Ref. | Ref. | Ref. |
| II | >0,32 | 22 | 244 | 8,30 | 1,1-62,46 | 0,013* |
*statistically significant result (p <0.05) PAT.237085
The above results are the subject of a publication sent for publication by Derkacz R. et al., Blood Cadmium Level and the Risk of Cancer in Women with BRCA1 Mutations, Cancers, 2021.[28]
Evaluation of the cancer risk in men
Men who have never smoked cigarettes and whose blood cadmium concentration is below 0.14 µg/l have a nearly 6-fold reduced risk of cancer compared to men with a blood cadmium concentration above 0.28 µg/l.
Cancer incidence depending on blood cadmium concentration in non-smoking men (quarters)
| Quartiles | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 0,03-0,14 | 4 | 260 | Ref. | Ref. | Ref. |
| II | 0,14-0,21 | 7 | 257 | 1,77 | 0,51-6,12 | 0,54 |
| III | 0,21-0,28 | 15 | 249 | 3,92 | 1,28-11,96 | 0,02 |
| IV | 0,28-2,34 | 21 | 242 | 5,64 | 1,91-16,67 | 0,0004* |
*statistically significant result (p<0.05) P.437897
Zinc is an essential element for the proper functioning of the body. It plays a protective role against free radicals, including being part of superoxide dismutase (SOD2). It is also involved in immune processes, contributes to the proper functioning of the skin and mucous membranes, and is involved in storing and secreting insulin from the pancreas. Zinc maintains the ion balance of other trace elements, including selenium, magnesium, and copper, and also plays a detoxifying role with heavy metals. Deficiency of this element leads to serious disorders such as immunodeficiencies, inflammation (including SARS-CoV-2), impaired wound healing, reduced fertility, and vision problems [29,30].
It has been observed that zinc levels change in cancer cells[31]. Normal prostate epithelial cells accumulate zinc, while in cancer cells the level of this element is significantly reduced.[32] Zinc is believed to have anti-cancer effects by inhibiting the growth of cancer cells and activating apoptosis. There are known studies assessing the relationship between zinc concentration and the risk of cancer, but their results are divergent. Some of these studies say that serum zinc concentration is higher in people with cancer [33-35], while others say that the level is lower.[36] The results of research conducted so far also suggest that an appropriate amount of zinc in the diet has a chemopreventive effect. People whose diet is rich in zinc have a lower risk of lung cancer than people following a zinc-poor diet (OR 0.71; 95% CI 0.5-0.99). [37] The risk of colorectal cancer is also lower with a zinc-rich diet (RR 0.86; 95% CI 0.73-1.02).[38] However, zinc supplementation in very high doses above 100 mg/day (the recommended daily intake of zinc is 8 mg/day for women and 12 mg/day for men) has the opposite effect, significantly increasing the risk of prostate cancer (RR 2.29; 95% CI 1.06 – 4.95, p=0.03).[39] The results of research conducted in our Center are presented below.
Evaluation of the cancer risk in women - BRCA1 non-carriers
Non-smoking women over 50 years of age with a zinc concentration in the blood in the range of 5600-6100 µg/l have a 6.5-fold reduced risk of cancer compared to women with a zinc concentration below 5600 µg/l, and a 3.5-fold reduced risk compared to women with zinc concentration above 6100 µg/l.
Incidence of cancer depending on the concentration of zinc in the blood in non-smoking women over 50 years of age.
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <5600 | 20 | 198 | 6,5 | 1-89-22,12 | 0,0008* |
| II | 5600-6100 | 3 | 192 | Ref. | Ref. | Ref. |
| III | >6100 | 22 | 399 | 3,5 | 1,04-11,94 | 0,029 |
*statistically significant result (p<0.05) P.437571
Evaluation of the cancer risk in women - BRCA1 carriers
Women with a mutation in the BRCA1 gene who do not smoke cigarettes have an almost 3-fold reduced risk of cancer if their zinc level is in the range of 6000-6700 µg/l. Such a correlation cannot be found among cigarette smokers.
Incidence of cancers depending on the concentration of zinc in the blood in women carriers of mutations in the BRCA1 gene who did not smoke cigarettes (selected ranges)
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 6000-6700 | 8 | 218 | Ref. | Ref. | Ref. |
| II | <6000&>6700 | 49 | 481 | 2,8 | 1,3-6,0 | 0,006* |
*statistically significant result (p<0.05) P.425603
Evaluation of the cancer risk in men
Men who have not smoked cigarettes have a nearly four-fold reduced risk of cancer if their blood zinc concentration is in the range of 5600-6350 µg/l.
Częstość występowania raków w zależności od stężenia cynku we krwi u mężczyzn niepalących (wybrane zakresy)
| Quartiles | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 5600-6350 | 5 | 318 | Ref. | Ref. | Ref. |
| II | <5600&>6350 | 41 | 687 | 3,8 | 1,5-9,7 | 0,0018* |
*statistically significant result (p<0.05) P.437894
Survival of patients with breast cancer
Women diagnosed with breast cancer and serum zinc concentration > 1000 µg/l have a nearly 6-fold reduced risk of death compared to women with zinc concentration < 700 µg/l.
The table shows the incidence of death according to serum zinc concentration in women with breast cancer (n=538).
Incidence of death depending on serum zinc concentration in women with breast cancer within 10 years of diagnosis (selected ranges)
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | ≤700 | 33 | 25 | 5,68 | 2,09-15,42 | 0,0003* |
| II | 700-850 | 181 | 54 | 2,24 | 0,91-5,53 | 0,088 |
| III | 851-1000 | 158 | 36 | 1,71 | 0,68-4,31 | 0,30 |
| IV | ≥1000 | 45 | 6 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05) P.434767
Survival of patients with prostate cancer
Men diagnosed with prostate cancer whose serum zinc concentration is in the range of 1000-1200 µg/l have an almost 4-fold reduced risk of death compared to men whose serum zinc concentration is < 900 µg/l.
Incidence of death depending on serum zinc concentration in men with prostate cancer within 5 years of diagnosis (quarters)
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | ≤760,38 | 56 | 34 | 8,4 | 3,31-21,33 | <0,0001* |
| II | 760,39-839,63 | 75 | 14 | 2,58 | 0,94-7,06 | 0,095 |
| III | 839,64-931,72 | 78 | 11 | 1,95 | 0,69-5,53 | 0,31 |
| IV | ≥931,73 | 83 | 6 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
Among men diagnosed with prostate cancer whose serum zinc concentration is in the range of 1000-1200, a nearly 4-fold reduced risk of death was observed compared to men whose zinc concentration is lower than 900 µg/l.
Incidence of death depending on serum zinc concentration in men with prostate cancer within 5 years of diagnosis (selected ranges)
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | <900 | 180 | 54 | 3,7 | 1,09-12,48 | 0,03* |
| II | 1000-1200 | 37 | 3 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The above results are the subject of patent application P.437046.
Survival of patients with laryngeal cancer
Among people with laryngeal cancer, the risk of death was more than twice reduced in patients with zinc concentrations above 688 µg/l in serum compared to those with concentrations <580 µg/l.
Frequency of deaths depending on serum zinc concentration in patients with laryngeal cancer within 5 years of diagnosis (tertiles)
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | 357,76-580,38 | 52 | 52 | 2,45 | 1,4-4,4 | <0,01* |
| II | 584,46-688,89 | 67 | 37 | 1,35 | 0,8-2,4 | 0,31 |
| III | 688,94-1317,87 | 76 | 31 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05) P.427370
The above results are the subject of the publication Lubiński J. et al., Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes, Biomolecules2021, 11, 865.[40]
Copper performs various functions in the structure of proteins and as a catalyst thanks to the ability to change the degree of oxidation and reduction and occurs in the oxidized (Cu2+) or reduced (Cu+) state. Copper ions can participate in a wide range of interactions with proteins, enabling the formation of complex structures and mediating complex biochemical reactions.[41,42] Copper, like other trace elements, is, on the one hand, essential for the body, but on the other hand, it is very dangerous. . In general, however, conditions that are characterized by general or cell-specific copper accumulation are rare and most often occur as a result of specific genetic disorders.[43]
Evaluation of the cancer risk in women - BRCA1 non-carriers
Non-smoking women under 50 with blood copper concentrations between 850-1000 µg/l have an almost three-fold reduced risk of cancer.
Incidence of cancer in non-smoking women under 50 years of age with no mutations in the BRCA1 gene (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 850-1000 | 16 | 493 | Ref. | Ref. | Ref. |
| II | <850 | 13 | 156 | 2,6 | 1,2-5,5 | 0,02* |
*statistically significant result (p<0.05) P.438572
Environmental lead contamination is an ongoing problem for developing societies. The toxic effect of lead mainly concerns its effect on the hematopoietic system[44], the peripheral and central nervous system[45] and the gastrointestinal tract[46]. Due to the ubiquity of lead, virtually every person is exposed to contact with this element. Lead toxicity leads, among other things, to changes in the activity of many enzymes and disturbances in the functions of free and structural proteins in the cell[47]. Many studies suggest that an important molecular mechanism of lead toxicity is its participation in the formation of free oxygen radicals, which play a large role in the formation of intracellular damage and in the pathogenesis of many diseases, including malignant tumors[48]. According to the IARC classification of carcinogens, lead and its compounds belong to groups 2a and 2b, i.e. potentially carcinogenic [12].
Evaluation of the cancer risk in women - BRCA1 non-carriers
Women with blood lead concentration ≤ 8 µg/l have a significantly reduced risk of developing cancer by almost 9 times.
Cancer incidence depends on lead concentration in women. (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | ≤8 | 1 | 372 | Ref. | Ref. | Ref. |
| II | >8 | 26 | 1094 | 8,84 | 1,2-65,41 | 0,006* |
*statistically significant result (p<0.05) P.433150
Evaluation of the cancer risk in women - BRCA1 carriers
Women with blood lead concentration ≤ 8 µg/l have a significantly reduced risk of developing cancer by three times.
Cancer incidence depends on lead concentration in women. (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | ≤8 | 8 | 217 | Ref. | Ref. | Ref. |
| II | >8 | 87 | 812 | 2,9 | 1,4-6,1 | 0,002* |
*statistically significant result (p<0.05) P.433150
Evaluation of the cancer risk in men
Men under 60 years of age and non-smokers have an almost 8-fold reduced risk of developing the disease if their lead concentration is lower than 13.5 µg/l.
Cancer incidence depends on lead concentration in non-smoking men under 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <13,5 | 1 | 217 | Ref. | Ref. | Ref. |
| II | >13,5 | 16 | 447 | 7,8 | 1,02-59,0 | 0,017* |
*statistically significant result (p<0.05) P.437899
Men over 60 years of age, non-smokers have a four-fold reduced risk of cancer if their lead concentration is in the range of 20-50 µg/l in whole blood.
Cancer incidence depends on lead concentration in non-smoking men over 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 20-50 | 3 | 74 | Ref. | Ref. | Ref. |
| II | <20 & >50 | 15 | 90 | 4,1 | 1,1-14,8 | 0,02* |
*statistically significant result (p<0.05) P.437899
Manganese (Mn) is an essential nutrient involved in properly functioning the immune system, regulation of blood sugar levels and cellular energy, reproduction, digestion, bone growth, blood clotting and homeostasis, and defense against reactive oxygen species. The functions performed by manganese metalloproteins include oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. [49] Mn acts as a cofactor for various enzymes, including arginase, glutamine synthetase (GS), pyruvate carboxylase, and Mn superoxide dismutase (Mn-SOD) [50]. Mn tends to accumulate in the liver, pancreas, bones, and brain [50].
The literature contains studies describing the correlation between serum Mn concentration and the risk of breast and colorectal cancer. However, the published studies are retrospective, which makes them unreliable for the conclusion that manganese is a marker of cancer risk. In our Center, we assessed the correlation between manganese concentration and survival of women with breast cancer.
Survival of patients with breast cancer
Women diagnosed with breast cancer, whose serum manganese concentration is in the range of 1-10 µg/l, have a significantly reduced risk of death by over 1.5 times compared to other women.
The incidence of death depends on serum manganese concentration in women with breast cancer within 10 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | 1,0-10,0 | 197 | 49 | Ref. | Ref. | Ref. |
| II | <1 & > 10 | 204 | 87 | 1,55 | 1,09-2,20 | 0,01* |
*statistically significant result (p<0.05) P.438040
Chromium (Cr) is an element widely distributed in the Earth's crust. This element in small amounts is necessary for the proper functioning of the body, playing a role in the metabolic processes of glucose, some proteins, and fats [51–53]. Increased doses of chromium may have a strong toxic effect, destructively affecting the liver, kidneys, and hematopoietic system, and causing cancer [51,54,55]. Cr(VI) has been found to have a strong mutagenic effect. The results of association studies indicate a relationship between the concentration of chromium in the blood and the risk of cancer in the head, neck, and oral cavity.
In our Center, work was carried out on the correlation between serum chromium concentration and survival of women diagnosed with breast cancer.
Survival of patients with breast cancer
Women diagnosed with breast cancer whose serum chromium concentration is in the range of 0.20-3.5 µg/l have a significantly reduced risk of death by almost two times.
Incidence of death depending on serum chromium concentration in women with breast cancer within 10 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | 0,20-3,5 | 202 | 54 | Ref. | Ref. | Ref. |
| II | <0,20 & > 3,5 | 199 | 82 | 1,8 | 1,28-2,54 | 0,0002* |
*statistically significant result (p<0.05) P.438042
Women who smoke cigarettes and have been diagnosed with breast cancer have a significantly reduced risk of death by almost 4.5 times if their serum chromium concentration is higher than 0.5 µg/l.
Incidence of death depending on serum chromium concentration in smokers with breast cancer within 10 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | <0,5 | 157 | 56 | 4,46 | 1,39-14,26 | 0,01* |
| II | ≥0,5 | 38 | 3 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05) P.438042
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology 2020, 28, 667–695, doi:10.1007/s10787-020-00690-x.
- Combs, G.F.; Clark, L.C.; Turnbull, B.W. An Analysis of Cancer Prevention by Selenium. Biofactors 2001, 14, 153–159, doi:10.1002/biof.5520140120.
- Reddy, V.N.; Giblin, F.J.; Lin, L.R.; Dang, L.; Unakar, N.J.; Musch, D.C.; Boyle, D.L.; Takemoto, L.J.; Ho, Y.S.; Knoernschild, T.; et al. Glutathione Peroxidase-1 Deficiency Leads to Increased Nuclear Light Scattering, Membrane Damage, and Cataract Formation in Gene-Knockout Mice. Invest Ophthalmol Vis Sci 2001, 42, 3247–3255.
- Kuria, A.; Fang, X.; Li, M.; Han, H.; He, J.; Aaseth, J.O.; Cao, Y. Does Dietary Intake of Selenium Protect against Cancer? A Systematic Review and Meta-Analysis of Population-Based Prospective Studies. Crit Rev Food Sci Nutr 2020, 60, 684–694, doi:10.1080/10408398.2018.1548427.
- Jenkins, D.J.A.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Blanco Mejia, S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.C.; et al. Selenium, Antioxidants, Cardiovascular Disease, and All-Cause Mortality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Clin Nutr 2020, 112, 1642–1652, doi:10.1093/ajcn/nqaa245.
- Schomburg, L. The Other View: The Trace Element Selenium as a Micronutrient in Thyroid Disease, Diabetes, and Beyond. Hormones (Athens) 2020, 19, 15–24, doi:10.1007/s42000-019-00150-4.
- Kenfield, S.A.; Van Blarigan, E.L.; DuPre, N.; Stampfer, M.J.; L Giovannucci, E.; Chan, J.M. Selenium Supplementation and Prostate Cancer Mortality. J Natl Cancer Inst 2015, 107, 360, doi:10.1093/jnci/dju360.
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.; Falco, M.; et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients 2021, 13, 953, doi:10.3390/nu13030953.
- Pietrzak, S.; Wójcik, J.; Scott, R.J.; Kashyap, A.; Grodzki, T.; Baszuk, P.; Bielewicz, M.; Marciniak, W.; Wójcik, N.; Dębniak, T.; et al. Influence of the Selenium Level on Overall Survival in Lung Cancer. J Trace Elem Med Biol 2019, 56, 46–51, doi:10.1016/j.jtemb.2019.07.010.
- Lener, M.R.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer. Cancer Res Treat 2016, 48, 1056–1064, doi:10.4143/crt.2015.282.
- Rogoża-Janiszewska E. et al Serum Selenium Level and the 10-Year Survival after Melanoma. Biomedicines SI: Role of Trace Elements in Chemoprevention and Cancer Therapy 2021.
- List of Classifications – IARC Monographs on the Identification of Carcinogenic Hazards to Humans Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 20 July 2021).
- Marciniak, W.; Derkacz, R.; Muszyńska, M.; Baszuk, P.; Gronwald, J.; Huzarski, T.; Cybulski, C.; Jakubowska, A.; Falco, M.; Dębniak, T.; et al. Blood Arsenic Levels and the Risk of Familial Breast Cancer in Poland. Int J Cancer 2020, 146, 2721–2727, doi:10.1002/ijc.32595.
- Marciniak, W.; Matoušek, T.; Domchek, S.; Paradiso, A.; Patruno, M.; Irmejs, A.; Roderte, I.; Derkacz, R.; Baszuk, P.; Kuświk, M.; et al. Blood Arsenic Levels as a Marker of Breast Cancer Risk among BRCA1 Carriers. Cancers 2021, 13, 3345, doi:10.3390/cancers13133345.
- Fowler, B.A. Monitoring of Human Populations for Early Markers of Cadmium Toxicity: A Review. Toxicol Appl Pharmacol 2009, 238, 294–300, doi:10.1016/j.taap.2009.05.004.
- Vinceti, M.; Venturelli, M.; Sighinolfi, C.; Trerotoli, P.; Bonvicini, F.; Ferrari, A.; Bianchi, G.; Serio, G.; Bergomi, M.; Vivoli, G. Case-Control Study of Toenail Cadmium and Prostate Cancer Risk in Italy. Sci Total Environ 2007, 373, 77–81, doi:10.1016/j.scitotenv.2006.11.005.
- Qayyum, M.A.; Shah, M.H. Comparative Study of Trace Elements in Blood, Scalp Hair and Nails of Prostate Cancer Patients in Relation to Healthy Donors. Biol Trace Elem Res 2014, 162, 46–57, doi:10.1007/s12011-014-0123-4.
- Pirincci, N.; Gecit, I.; Gunes, M.; Kaba, M.; Tanik, S.; Yuksel, M.B.; Arslan, H.; Demir, H. Levels of Serum Trace Elements in Renal Cell Carcinoma Cases. Asian Pac J Cancer Prev 2013, 14, 499–502, doi:10.7314/apjcp.2013.14.1.499.
- Kellen, E.; Zeegers, M.P.; Hond, E.D.; Buntinx, F. Blood Cadmium May Be Associated with Bladder Carcinogenesis: The Belgian Case-Control Study on Bladder Cancer. Cancer Detect Prev 2007, 31, 77–82, doi:10.1016/j.cdp.2006.12.001.
- Wolf, C.; Strenziok, R.; Kyriakopoulos, A. Elevated Metallothionein-Bound Cadmium Concentrations in Urine from Bladder Carcinoma Patients, Investigated by Size Exclusion Chromatography-Inductively Coupled Plasma Mass Spectrometry. Anal Chim Acta 2009, 631, 218–222, doi:10.1016/j.aca.2008.10.035.
- Farzin, L.; Moassesi, M.E.; Sajadi, F.; Ahmadi Faghih, M.A. Evaluation of Trace Elements in Pancreatic Cancer Patients in Iran. Middle East Journal of Cancer 2013, 4, 79–86.
- Amaral, A.F.S.; Porta, M.; Silverman, D.T.; Milne, R.L.; Kogevinas, M.; Rothman, N.; Cantor, K.P.; Jackson, B.P.; Pumarega, J.A.; López, T.; et al. Pancreatic Cancer Risk and Levels of Trace Elements. Gut 2012, 61, 1583–1588, doi:10.1136/gutjnl-2011-301086.
- Wu, H.-D.I.; Chou, S.-Y.; Chen, D.-R.; Kuo, H.-W. Differentiation of Serum Levels of Trace Elements in Normal and Malignant Breast Patients. Biol Trace Elem Res 2006, 113, 9–18, doi:10.1385/BTER:113:1:19.
- Deeb, M.; El-Sheredy, H.; Mohammed, A. The Role of Serum Trace Elements and Oxidative Stress in Egyptian Breast Cancer Patients. Advances in Breast Cancer Research 2016, 05, 37–47, doi:10.4236/abcr.2016.51004.
- Sabir, S.; Akash, M.S.H.; Fiayyaz, F.; Saleem, U.; Mehmood, M.H.; Rehman, K. Role of Cadmium and Arsenic as Endocrine Disruptors in the Metabolism of Carbohydrates: Inserting the Association into Perspectives. Biomed Pharmacother 2019, 114, 108802, doi:10.1016/j.biopha.2019.108802.
- Lee, J.-E.; Kim, H.-R.; Lee, M.; Kim, N.-H.; Wang, K.-M.; Lee, S.; Park, O.; Hong, E.-J.; Youn, J.-W.; Kim, Y.-Y. Smoking-Related DNA Methylation Is Differentially Associated with Cadmium Concentration in Blood. Biochem Genet 2020, 58, 617–630, doi:10.1007/s10528-020-09965-y.
- Bolam, T.; Bersuder, P.; Burden, R.; Shears, G.; Morris, S.; Warford, L.; Thomas, B.; Nelson, P. Cadmium Levels in Food Containing Crab Brown Meat: A Brief Survey from UK Retailers. Journal of Food Composition and Analysis 2016, 54, 63–69, doi:10.1016/j.jfca.2016.10.005.
- Derkacz R. et al. Blood Cadmium Level and the Risk of Cancer in Women with BRCA1 Mutations. Cancers, SI: Advances in Inherited Breast and Ovarian Cancer and Its Imaging 2021.
- Nasiadek, M.; Stragierowicz, J.; Klimczak, M.; Kilanowicz, A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients 2020, 12, 2464, doi:10.3390/nu12082464.
- Puzanowska-Tarasiewicz, H.; Kuźmicka, L.; Tarasiewicz, M. Funkcje biologiczne wybranych pierwiastków i ich związków. 3, 3,. Polski Merkuriusz Lekarski : organ Polskiego Towarzystwa Lekarskiego. 2009, 419–422.
- To, P.K.; Do, M.H.; Cho, J.-H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int J Mol Sci 2020, 21, E2991, doi:10.3390/ijms21082991.
- Zaichick VYe, null; Sviridova, T.V.; Zaichick, S.V. Zinc in the Human Prostate Gland: Normal, Hyperplastic and Cancerous. Int Urol Nephrol 1997, 29, 565–574, doi:10.1007/BF02552202.
- Siddiqui, M.K.J.; Jyoti, null; Singh, S.; Mehrotra, P.K.; Singh, K.; Sarangi, R. Comparison of Some Trace Elements Concentration in Blood, Tumor Free Breast and Tumor Tissues of Women with Benign and Malignant Breast Lesions: An Indian Study. Environ Int 2006, 32, 630–637, doi:10.1016/j.envint.2006.02.002.
- Pasha, Q.; Malik, S.A.; Shah, M.H. Statistical Analysis of Trace Metals in the Plasma of Cancer Patients versus Controls. J Hazard Mater 2008, 153, 1215–1221, doi:10.1016/j.jhazmat.2007.09.115.
- el-Ahmady, O.; el-Maraghy, A.; Ibrahim, A.; Ramzy, S. Serum Copper, Zinc, and Iron in Patients with Malignant and Benign Pulmonary Diseases. Nutrition 1995, 11, 498–501.
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol Trace Elem Res 2002, 89, 1–11, doi:10.1385/BTER:89:1:1.
- Zhou, W.; Park, S.; Liu, G.; Miller, D.P.; Wang, L.I.; Pothier, L.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Christiani, D.C. Dietary Iron, Zinc, and Calcium and the Risk of Lung Cancer. Epidemiology 2005, 16, 772–779, doi:10.1097/01.ede.0000181311.11585.59.
- Zhang, X.; Giovannucci, E.L.; Smith-Warner, S.A.; Wu, K.; Fuchs, C.S.; Pollak, M.; Willett, W.C.; Ma, J. A Prospective Study of Intakes of Zinc and Heme Iron and Colorectal Cancer Risk in Men and Women. Cancer Causes Control 2011, 22, 1627–1637, doi:10.1007/s10552-011-9839-z.
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc Supplement Use and Risk of Prostate Cancer. J Natl Cancer Inst 2003, 95, 1004–1007, doi:10.1093/jnci/95.13.1004.
- Lubiński, J.; Jaworowska, E.; Derkacz, R.; Marciniak, W.; Białkowska, K.; Baszuk, P.; Scott, R.J.; Lubiński, J.A. Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes. Biomolecules 2021, 11, 865, doi:10.3390/biom11060865.
- Festa, R.A.; Thiele, D.J. Copper: An Essential Metal in Biology. Curr Biol 2011, 21, R877-883, doi:10.1016/j.cub.2011.09.040.
- Ceramella, J.; Mariconda, A.; Iacopetta, D.; Saturnino, C.; Barbarossa, A.; Caruso, A.; Rosano, C.; Sinicropi, M.S.; Longo, P. From Coins to Cancer Therapy: Gold, Silver and Copper Complexes Targeting Human Topoisomerases. Bioorg Med Chem Lett 2020, 30, 126905, doi:10.1016/j.bmcl.2019.126905.
- Linder, M.C. The Relationship of Copper to DNA Damage and Damage Prevention in Humans. Mutat Res 2012, 733, 83–91, doi:10.1016/j.mrfmmm.2012.03.010.
- Johnson, F.M. The Genetic Effects of Environmental Lead. Mutat Res 1998, 410, 123–140, doi:10.1016/s1383-5742(97)00032-x.
- Marchetti, C. Molecular Targets of Lead in Brain Neurotoxicity. Neurotox Res 2003, 5, 221–236, doi:10.1007/BF03033142.
- Tomczyk, J.; Lewczuk, E.; Abdrzejak, R. Ostre Zatrucia Organicznymi Związkammi Ołowiu.; Medycyna Pracy, 1999; Vol. 50;.
- Cellular Mechanisms of Lead Neurotoxicity – PubMed Available online: https://pubmed.ncbi.nlm.nih.gov/16501435/ (accessed on 20 July 2021).
- Nersesyan, A.; Kundi, M.; Waldherr, M.; Setayesh, T.; Mišík, M.; Wultsch, G.; Filipic, M.; Mazzaron Barcelos, G.R.; Knasmueller, S. Results of Micronucleus Assays with Individuals Who Are Occupationally and Environmentally Exposed to Mercury, Lead and Cadmium. Mutat Res 2016, 770, 119–139, doi:10.1016/j.mrrev.2016.04.002.
- Aschner, M.; Erikson, K. Manganese. Adv Nutr 2017, 8, 520–521, doi:10.3945/an.117.015305.
- Chen, P. Manganese Metabolism in Humans. Front Biosci 2018, 23, 1655–1679, doi:10.2741/4665.
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and Oxidative Mechanisms of Different Forms of Chromium. Toxicology 2002, 180, 5–22, doi:10.1016/s0300-483x(02)00378-5.
- Cefalu, W.T.; Hu, F.B. Role of Chromium in Human Health and in Diabetes. Diabetes Care 2004, 27, 2741–2751, doi:10.2337/diacare.27.11.2741.
- Vincent, J.B. The Biochemistry of Chromium. J Nutr 2000, 130, 715–718, doi:10.1093/jn/130.4.715.
- Anderson, R.A. Chromium Metabolism and Its Role in Disease Processes in Man. Clin Physiol Biochem 1986, 4, 31–41.
- Anderson, R.A. Chromium as an Essential Nutrient for Humans. Regul Toxicol Pharmacol 1997, 26, S35-41, doi:10.1006/rtph.1997.1136.
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum Selenium Levels Predict Survival after Breast Cancer. Breast Cancer Res Treat 2018, 167, 591–598, doi:10.1007/s10549-017-4525-9.
- Lubiński, J.; Marciniak, W.; Muszynska, M.; Jaworowska, E.; Sulikowski, M.; Jakubowska, A.; Kaczmarek, K.; Sukiennicki, G.; Falco, M.; Baszuk, P.; et al. Serum Selenium Levels and the Risk of Progression of Laryngeal Cancer. PLoS One 2018, 13, e0184873, doi:10.1371/journal.pone.0184873.
Home » Research
Research
Research projects
Prevention of Hereditary Breast Cancer through Personalized Optimization of Se, Zn, Fe Levels in the Body Using Dietary Supplements
Agreement No. INNOMED/I/16/NCBR/2014
Project Duration as per the Agreement: 01.10.2014-30.09.2019. The project co-financed by the European Union under the European Regional Development Fund
The project partner is the West Pomeranian University of Technology in Szczecin
Characteristics of the studied groups
The study groups, whose analyses form the basis for the results described below, were created from individuals registered in the Hereditary Cancer Center in Szczecin between 2009 and 2020. Each patient provided informed consent for the storage and use of biological material for scientific purposes. Blood and serum samples were collected between 8 AM and 2 PM, and patients fasted for at least 4 hours before collection. For the majority of patients, a sample was collected only once, but in some cases, multiple samples were taken during subsequent visits. Biological material was stored at -80°C until the element concentrations were determined. Each participant in the study completed a health and lifestyle questionnaire.
A total of 2962 healthy women were included in the prospective cohort. During 42 months of follow-up, 148 women were diagnosed with cancer.
The characteristics of the group are presented in the table.
| Cases (n=148) | Healthy (n=2814) | |
| Average age (range) | 56,46 (35-82) | 53 (33-84) |
Smoking current former never | 40 (27,03%) 34 (22,97%) 74 (50 %) | 605 (21,50%) 750 (26,65%) 1459 (51,84%) |
Hormone replacement therapy / oral contraceptives no yes missing data | 93 (62,84%) 54 (36,49%) 1 (0,67%) | 1467 (52,13%) 1316 (46,77%) 31 (1,1%) |
Adnexektomia no yes missing data | 135 (91,22%) 9 (6,08%) 4 (2,7%) | 2631 (93,50%) 175 (6,22%) 8 (0,28%) |
Cancers (location) breast uterus colon melanoma thyroid ovary cervix lymphoma bladder lung kidney stomach leukemia myeloma glioblastoma pancreas |
76 (51,4%) 12 (8,1%) 10 (6,8%) 7 (4,7%) 7 (7,73%) 6 (4,05%) 5 (3,38%) 5 (3,38%) 4 (2,7%) 4 (2,7%) 3 (2,03%) 3 (2,03%) 2 (1,35%) 2 (1,35%) 1 (0,68%) 1 (0,68%) | – – – – – – – – – – – – – – – – |
| Cases (n=107) | Healthy (n=1217) | |
| Average age (range) | 44,25 (26-66) | 40,5 (25-69) |
| Smoking
current former never missing data |
24 (22,43%) 27 (25,23%) 55 (51,4%) 1 (0,93%) |
254 (20,87%) 249 (20,46%) 699 (57,44%) 15 (1,23%) |
| Hormone replacement therapy / oral contraceptives
no yes missing data |
46 (43%) 60 (56,07%) 1 (0,93%) |
603 (49,55%) 592 (48,64%) 22 (1,81%) |
| Adnexektomia
no yes missing data |
52 (48,6%) 55 (51,4%) 0 |
688 (56,53%) 510 (41,92%) 19 (1,56%) |
| Cancers (location)
breast ovary cervix peritoneum stomach colon leukemia pancreas skin larynx thyroid bladder |
80 (74,8%) 15 (14%) 3 (2,8%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) |
– – – – – – – – – – – – |
| Cases (n=144) | Healthy (n=2812) | |
| Average age (range) | 60,5 (36-76) | 52 (31-87) |
| Smoking
current former never |
42 (29,17%) 55 (38,19%) 47 (32,64 %) |
829 (29,48%) 975 (34,67%) 1008 (35,85%) |
| Cancers (location)
prostate skin kidney colon bladder blood lung liver thyroid pancreas stomach breast esophagus pituitary gland salivary glands testis |
58 (40,28%) 16 (11,11%) 13 (9,03%) 13 (9,03%) 12 (8,33%) 9 (6,25%) 6 (4,17%) 4 (2,78%) 4 (2,78%) 2 (1,39%) 2 (1,39%) 1 (0,69%) 1 (0,69%) 1 (0,69%) 1 (0,69%) 1 (0,69%) |
– – – – – – – – – – – – – – – – |
| Alive (n=417) | Deaths (n=121) | |
| Average age (range) | 55,87 (25-85) | 61,07 (28-91) |
| Smoking
current former never missing data |
89 (21,3%) 112 (26,9%) 206 (49,4%) 10 (2,4%) |
26 (21,5%) 27 (22,3%) 65 (53,7%) 3 (2,5%) |
| Estrogen receptor
positive (ER+) negative (ER-) missing data |
287 (68,8%) 114 (27,3%) 16 (3,8%) |
79 (65,3%) 32 (26,4%) 10 (8,26%) |
| BRCA1 mutation | 50 (12%) | 11 (9,1%) |
| Chemotherapy
yes no missing data |
211 (50,6%) 174 (41,7%) 32 (7,7%) |
65 (53,7%) 36 (29,8%) 20 (16,5%) |
| Radiotherapy
yes no missing data |
244 (58,5%) 122 (29,3%) 51 (12,2%) |
56 (46,3%) 35 (28,9%) 30 (24,8%) |
| Tamoxifen
yes no missing data |
282 (67,6%) 122 (29,3%) 13 (3,1%) |
79 (65,3%) 33 (27,3%) 9 (7,4%) |
| Type of operation
mastectomy lumpectomy missing data |
261 (62,6%) 131 (31,4%) 25 (6%) |
82 (67,8%) 17 (14%) 22 (18,2%) |
The study included 357 patients with diagnosed and histopathologically confirmed prostate cancer. Blood was collected between 2009 and 2015 at the time of diagnosis of prostate cancer before treatment. The average follow-up period for patients was 5 years.
| Gleason scale | Alive (n=293) | Deaths (n=102) |
6 7 8 9 10 missing data | 85 (29%) 164 (56%) 20 (6,8%) 17 (5,8%) 2 (0,7%) 5 (1,7%) | 39 (38,2%) 35 (34,3%) 9 (8,8%) 11 (10,8%) 7 (6,9%) 2 (1,96%) |
| Clinical stage 1-2 3 4 missing data | 7 (2,7%) 232 (79,2%) 51 (17,4%) 2 (0,7%) | 4 (3,9%) 45 (44,1%) 53 (52%) – |
| Radiotherapy yes no | 84 (28,7%) 209 (71,3%) | 24 (23,5%) 78 (76,5%) |
| Chemotherapy yes no | 3 (1%) 290 (99%) | 4 (3,9%) 98 (96,1%) |
| Hormonetherapy yes no | 59 (20,1%) 234 (79,9%) | 40 (39,2%) 62 (60,8%) |
| Prostatectomy yes no | 233 (79,5%) 60 (20,5%) | 33 (32,4%) 69 (67,6%) |
| Orchidectomy yes no | 8 (2,7%) 285 (97,3%) | 11 (10,8%) 91 (89,2%) |
| n=315 | % | |
| Average age of cancer diagnosis (range) | 61,1 (41-86) | |
| Sex
women men |
49 266 |
15,6 84,4 |
| Clinical stage
1 2 3 4 |
72 42 70 131 |
22,8 13,3 22,2 41,6 |
| Pack-years (average, range) | 37,11 (0-150) | |
| Treatment
radiotherapy chemotherapy |
142 32 |
45,1 10,1 |
| % | ||
| Sex
Men Women |
196 106 |
64,9 35,1 |
| Average age (range) | 64,2 (43-86) | – |
| Pack-years (range) | 33,2 (0-232,8) | – |
| Smoking
yes no |
283 19 |
93,7 6,3 |
| Clinical stage
1 2 3 4 |
129 75 79 19 |
42,7 24,8 26,2 6,3 |
| Radiotherapy
yes no |
78 224 |
25,8 74,2 |
| Chemotherapy
yes no |
105 197 |
34,8 65,2 |
| Histological type
Adenocarcinoma Squamous cell carcinoma Large cell cancer Large and Small Cell Mixed Carcinoma Small Cell Carcinoma Other |
136 124 21 5 3 13 |
45 41,1 7 1,7 1 4,3 |
| New cancer diagnosis | |
| Year of birth (range) | 1930-1976 |
| Average age at the blood donation (range) | 63,4 (35-84) |
| Sex
men women |
59 41 |
| First-Degree Relatives
with pancreatic cancer with other cancer |
4 43 |
| Smoking
yes no |
27 73 |
| Pack-years (range) | 28,99 (2-50) |
| Alive (n=344) | Deaths (n=31) | |
| Average age (range) | 54,6 (21-90) | 64,3 (38-86) |
| Sex
women men |
218 (63%) 126 (37%) |
14 (45%) 17 (55%) |
| Clinical stage
2 3 4-5 |
70 (20%) 145 (42%) 129 (38%) |
1 (3,2%) 12 (39%) 18 (58%) |
Research results
In the body, selenium acts through proteins into which it is incorporated in the form of selenocysteine. As a component of selenoproteins, selenium plays an enzymatic and structural role[1]. Some of the most important functions of selenoproteins include participation in the production of thyroid hormones, stimulation of the immune system, and protection against oxidative stress.[2]
Both deficiency and excess of this element may harm the body. This leads to, among others: heart disorders, heart and liver degeneration, increased risk of hypertensive disease, reduced immune system efficiency, thyroid dysfunction, impaired bone mineralization and proper tooth formation, and increased risk of cancer. [3–7]
Evaluation of the cancer risk in women - BRCA1 non-carriers
Women over 60 years old and non-smokers have a significantly 4.5-fold reduced risk if their blood concentration is in the range of 94-104 µg/l. However, women over 60 years of age who currently smoke or have smoked in the past have a significantly 3.5-fold reduced risk of cancer if their blood selenium concentration is > 110 µg/l.
Incidence of cancer in non-smoking women over 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 94-104 | 3 | 114 | Ref. | Ref. | Ref. |
| II | <94 | 11 | 93 | 4,5 | 1,2-16,6 | 0,02* |
*statistically significant result (p<0.05) P.438038
Incidence of cancer in smoking women over 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | >110 | 4 | 97 | Ref. | Ref. | Ref. |
| II | <110 | 25 | 171 | 3,5 | 1,2-10,5 | 0,02* |
*statistically significant result (p<0.05) P.438038
Evaluation of the cancer risk in women - BRCA1 carriers
Women under 50 years of age with blood selenium concentrations in the range of 70-80 µg/l tend to have a nearly 5-fold reduced risk of cancer compared to women with selenium concentrations outside the presented range.
Cancer incidence depending on blood selenium concentration among women under 50 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 70-80 | 1 | 56 | Ref. | Ref. | Ref. |
| II | <70 & >80 | 75 | 904 | 4,6 | 0,6-34,1 | 0,1* |
*result not statistically significant (p >0.05) P.435603
Women over 50 years of age with blood selenium concentrations in the range of 95-120 µg/l have a more than twofold reduced risk of cancer compared to women with selenium concentrations outside the presented range.
Cancer incidence depending on blood selenium concentration among women over 50 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 95-120 | 13 | 156 | Ref. | Ref. | Ref. |
| II | <95 & >120 | 20 | 102 | 2,3 | 1,1-4,9 | 0,03* |
*statistically significant result (p <0.05) P.435603
Evaluation of the cancer risk in men
Men who do not smoke cigarettes and have selenium concentrations in the blood range of 100-110 µg/l have a nearly 4-fold reduced risk of cancer compared to men with selenium concentrations outside this range.
Cancer incidence depending on blood selenium concentration among non-smoking men (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 100-110 | 5 | 260 | Ref. | Ref. | Ref. |
| II | <95 | 20 | 291 | 3,6 | 1,3-9,7 | 0,008* |
*statistically significant result (p 115 µg/l , compared to men with blood selenium concentration <115 µg/l.
Cancer incidence depends on blood selenium concentration among smoking men over 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <115 | 1 | 83 | Ref. | Ref. | Ref. |
| II | ≥115 | 56 | 427 | 10,9 | 1,5-79,8 | 0,0013* |
*statistically significant result (p < 0.05) P.437898
The Kaplan-Meier curve for the above correlation is shown below.

-
-
Survival of patients with breast cancer
Women with breast cancer have a significantly greater chance of 10-year survival if their serum selenium concentration is >94.7 µg/l. Women with serum selenium concentration < 70 µg/l have a more than 25-fold increased risk of death compared to the subgroup with serum selenium concentration 95-102.5 µg/l.
Incidence of death depending on serum selenium concentration in women with breast cancer within 10 years of diagnosis (quarters).
| Quartiles | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | 52,1-76,7 | 89 | 46 | 2,35 | 1,12-4,55 | 0,01* |
| II | 76,8-85,1 | 105 | 29 | 1,52 | 0,76-3,02 | 0,23 |
| III | 85,2-94,6 | 105 | 29 | 1,95 | 1,01-3,76 | 0,047* |
| IV | 94,7-171,5 | 118 | 17 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The incidence of death depends on serum selenium concentration in women with breast cancer within 10 years of diagnosis (ranges).
| Quartiles | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | < 70 | 32 | 28 | Ref. | Ref. | Ref. |
| II | 95-102,5 | 62 | 2 | 27,1 | 6,7-121,2 | <0,0001* |
*statistically significant result (p<0.05)
The above results are part of the publication by Szwiec M et al., Serum Selenium Level Predicts 10-Years Survival after Breast Cancer; Nutrients 2021, 13(3), 953. [8]
Survival of patients with prostate cancer
Men with prostate cancer and serum selenium concentration in the range of 85-105 µg/l have a significantly reduced risk of death by over 8 times compared to the subgroup with serum selenium concentration below 70 µg/l.
The incidence of death depends on serum selenium concentration in men with prostate cancer within 5 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | ≤70 | 61 | 33 | 8,6 | 3,4-21,7 | <0,0001* |
| II | 85-105 | 95 | 6 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
Survival of patients with lung cancer
It has been observed that people whose serum selenium concentration is >67.4 µg/l have a greater chance of longer survival after a lung cancer diagnosis than people with a low serum selenium concentration (<55.1 µg/l).
Frequency of deaths depending on serum selenium concentration in patients with stage I lung cancer within 3 years of diagnosis (tertiles).
| Tertiles | Range [µg/l] | total | OR | 95%CI | p |
| I | 33,46-57,91 | 43 | 2,73 | 1,21-6,11 | 0,01* |
| II | 57,92-68,86 | 42 | 1,88 | 0,83-4,28 | 0,13 |
| III | 69,29-108,27 | 44 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The above results are part of the publication by Pietrzak S et al., Influence of the selenium level on overall survival in lung cancer, Journal of Trace Elements in Medicine and Biology 2019, 56:46-51.[9]
Survival of patients with pancreatic cancer
Patients diagnosed with pancreatic cancer have a 3-fold greater chance of 6-month survival if their serum selenium concentration is ≥ 63.67 µg/l compared to patients with selenium concentration below this value.
The incidence of death depends on serum selenium concentration in patients with pancreatic cancer within 6 months of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | >< 63,67 | 24 | 33 | 0,4 | 0,1-0,97 | 0,03* |
| II | ≥ 63,67 | 22 | 11 | Ref. | Ref. | Ref. |
The above results are part of the publication by Lener M et al., Serum concentration of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer, Cancer Res Treat., 2016, 48(3):1056-1064.[10]
Survival of patients with melanoma
Patients diagnosed with malignant melanoma have a significantly greater chance of 10-year survival if their serum selenium concentration is higher than 96 µg/l. Patients with serum selenium concentration 96 µg/l.
The incidence of death depends on serum selenium concentration in patients with malignant melanoma within 10 years of diagnosis (quarters).
| Quartiles | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | 56,7-76,2 | 78 | 16 | 5,83 | 1,32-25,8 | 0,02* |
| II | 76,4-85,01 | 86 | 7 | 3,37 | 0,7-16,3 | 0,13 |
| III | 85,15-96,06 | 88 | 6 | 3,34 | 0,67-16,7 | 0,14 |
| IV | 96,15-168 | 92 | 2 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The above results are the subject of the publication by Rogoża-Janiszewska E. et al., Serum selenium level and the 10-year survival after melanoma, Biomedicines, 2021.[11]
Arsenic and its compounds are among the most recognized toxins. According to the classification of the International Agency for Research on Cancer (IARC), arsenic and its compounds have been categorized as Group 1[12] carcinogens, meaning they are definite human carcinogens. The clinical manifestations resulting from inhalation or ingestion of arsenic compounds vary widely. Depending on the concentration, duration of exposure, and route of absorption, the effects of arsenic interaction with tissues range from relatively benign, such as hypopigmentation, to life-threatening tumors (WHO). Based on existing literature, it can be concluded that not only high but also slightly elevated arsenic concentrations may be linked to cancers, especially in women.
Evaluation of the cancer risk in women - BRCA1 non-carriers
Women with an arsenic concentration in blood below 0.6 µg/l have a significant, almost 5-fold reduced risk of developing cancers, especially breast cancer, compared to women with an arsenic concentration above 0.6 µg/l (OR=4.7; p=0 .0004; 95%CI:1.9-11.7).
Incidence of breast cancer depending on arsenic concentration in blood (selected ranges)
| Group | Range | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <0,6 | 5 | 735 | Ref. | Ref. | Ref. |
| II | 0,6-0,81 | 18 | 723 | 3,7 | 1,4-10 | 0,01* |
| III | 0,82-1,25 | 21 | 719 | 4,3 | 1,6-11-5 | 0,002* |
| IV | >1,25 | 32 | 709 | 6,6 | 2,6-17,1 | <0,0001* |
| Selected ranges | ||||||
| I | <0,6 | 5 | 735 | Ref. | Ref. | Ref. |
| II | ≥0,6 | 71 | 2222 | 4,7 | 1,9-11,7 | 0,0004* |
*statistically significant result (p<0.05) P.425602
The Kaplan-Meier curve for the above correlation is shown below.

-
The above results are the subject of the publication Marciniak W. et al., Blood arsenic levels and the risk of familial breast cancer in Poland, Int J Cancer, 2020, 146 (10): 2721-2727.[13]
Evaluation of the cancer risk in women - BRCA1 carriers
Women with an arsenic concentration in blood below 0.85 µg/l have a significantly reduced risk of developing cancer by approximately two times compared to women with an arsenic concentration above 0.85 µg/l (OR=2.55; p=0.0006; 95% CI:1.47-4.43).
The incidence of cancer depends on the concentration of arsenic in the blood.
| Group | Range | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <0,85 | 18 | 513 | Ref. | Ref. | Ref. |
| II | >0,85 | 49 | 548 | 2,55 | 1,47-4,43 | 0,0006* |
*statistically significant result (p<0.05)
The above results are part of the publication by Marciniak W. et al., Blood Arsenic Levels as a Marker of Breast Cancer Risk among BRCA1 Carriers, Cancers 2021, 13(13), 3345. [14]
Evaluation of the cancer risk in men
Men whose blood arsenic concentration is between 0.7 and 1.14 µg/l have a nearly 5-fold reduced risk of cancer.
Cancer incidence in men depending on arsenic concentration in blood (selected ranges)
| Group | Range [µg/l] | .New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 0,7-1,14 | 28 | 788 | Ref. | Ref. | Ref. |
| II | <0,7 & > 1,14 | 116 | 2003 | 4,8 | 1,1-2,5 | 0,03* |
*statistically significant result (p<0.05) P.437896
Survival of patients with prostate cancer
Men with serum arsenic concentration in the range of 0.7-1.0 µg/l have a significantly reduced risk of death by more than 3 times compared to the subgroup with serum arsenic concentration < 0.7 µg/l.
The incidence of death depends on serum arsenic concentration in men with prostate cancer within 5 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Deaths | Alive | OR | 95%CI | p |
| I | <0,7 | 22 | 50 | 3,18 | 1,63-6,22 | 0,0009* |
| II | 0,7-1,0 | 22 | 159 | Ref. | Ref. | Ref. |
| III | >1,0 | 21 | 83 | 1,83 | 0,95-3,52 | 0,085 |
*statistically significant result (p<0.05)
According to the classification of the International Agency for Research on Cancer (IARC), cadmium and its compounds have been categorized as Group 1 [12] human carcinogens. The adverse effects of cadmium and its compounds can lead to kidney diseases, cardiovascular diseases, hypertension, anemia, liver damage, disorders of the reproductive organs, immune system disorders, deficiencies of iron, copper, and zinc, as well as the development of cancer. Numerous studies describe increased levels of cadmium in the biological material of individuals who have developed malignant tumors of the prostate, kidney, bladder, pancreas, and breast.
The literature mentions three main sources of cadmium: diet, smoking, and occupational exposure[25]. The concentration of cadmium in food products is strongly dependent on the content of this element in the environment - air, soil, and water[25]. The concentration of cadmium in the blood is strongly correlated with smoking tobacco products. In non-smokers, Cd concentration is lower compared to smokers[26]. The occupational group most exposed to cadmium are workers in the zinc, steel, and copper industries, as well as in the production of nickel-cadmium batteries, solar cells, and jewelry[27].
Evaluation of the cancer risk in women - BRCA1 non-carriers
The table below shows the distribution of subjects in our chosen range. The risk of cancer is reduced more than 8 times among women whose concentration is in the range of 0.28-0.33 µg/l.
Cancer incidence depending on blood cadmium concentration in non-smoking women over 50 years of age (selected range)
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 0,28-0,33 | 1 | 126 | Ref. | Ref. | Ref. |
| II | <0,28 & >0,33 | 44 | 665 | 8,34 | 1,1-61,1 | 0,009* |
*statistically significant result (p<0.05) P.437608
Evaluation of the cancer risk in women - BRCA1 carriers
In women under 51 years of age with blood cadmium concentration ≤0.32 µg/l, the risk is reduced by more than 8 times compared to women with higher cadmium levels.
Cancer incidence depending on blood cadmium concentration in women under 51 years of age (selected range)
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | ≤0,32 | 1 | 92 | Ref. | Ref. | Ref. |
| II | >0,32 | 22 | 244 | 8,30 | 1,1-62,46 | 0,013* |
*statistically significant result (p <0.05) PAT.237085
The above results are the subject of a publication sent for publication by Derkacz R. et al., Blood Cadmium Level and the Risk of Cancer in Women with BRCA1 Mutations, Cancers, 2021.[28]
Evaluation of the cancer risk in men
Men who have never smoked cigarettes and whose blood cadmium concentration is below 0.14 µg/l have a nearly 6-fold reduced risk of cancer compared to men with a blood cadmium concentration above 0.28 µg/l.
Cancer incidence depending on blood cadmium concentration in non-smoking men (quarters)
| Quartiles | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 0,03-0,14 | 4 | 260 | Ref. | Ref. | Ref. |
| II | 0,14-0,21 | 7 | 257 | 1,77 | 0,51-6,12 | 0,54 |
| III | 0,21-0,28 | 15 | 249 | 3,92 | 1,28-11,96 | 0,02 |
| IV | 0,28-2,34 | 21 | 242 | 5,64 | 1,91-16,67 | 0,0004* |
*statistically significant result (p<0.05) P.437897
Zinc is an essential element for the proper functioning of the body. It plays a protective role against free radicals, including being part of superoxide dismutase (SOD2). It is also involved in immune processes, contributes to the proper functioning of the skin and mucous membranes, and is involved in storing and secreting insulin from the pancreas. Zinc maintains the ion balance of other trace elements, including selenium, magnesium, and copper, and also plays a detoxifying role with heavy metals. Deficiency of this element leads to serious disorders such as immunodeficiencies, inflammation (including SARS-CoV-2), impaired wound healing, reduced fertility, and vision problems [29,30].
It has been observed that zinc levels change in cancer cells[31]. Normal prostate epithelial cells accumulate zinc, while in cancer cells the level of this element is significantly reduced.[32] Zinc is believed to have anti-cancer effects by inhibiting the growth of cancer cells and activating apoptosis. There are known studies assessing the relationship between zinc concentration and the risk of cancer, but their results are divergent. Some of these studies say that serum zinc concentration is higher in people with cancer [33-35], while others say that the level is lower.[36] The results of research conducted so far also suggest that an appropriate amount of zinc in the diet has a chemopreventive effect. People whose diet is rich in zinc have a lower risk of lung cancer than people following a zinc-poor diet (OR 0.71; 95% CI 0.5-0.99). [37] The risk of colorectal cancer is also lower with a zinc-rich diet (RR 0.86; 95% CI 0.73-1.02).[38] However, zinc supplementation in very high doses above 100 mg/day (the recommended daily intake of zinc is 8 mg/day for women and 12 mg/day for men) has the opposite effect, significantly increasing the risk of prostate cancer (RR 2.29; 95% CI 1.06 – 4.95, p=0.03).[39] The results of research conducted in our Center are presented below.
Evaluation of the cancer risk in women - BRCA1 non-carriers
Non-smoking women over 50 years of age with a zinc concentration in the blood in the range of 5600-6100 µg/l have a 6.5-fold reduced risk of cancer compared to women with a zinc concentration below 5600 µg/l, and a 3.5-fold reduced risk compared to women with zinc concentration above 6100 µg/l.
Incidence of cancer depending on the concentration of zinc in the blood in non-smoking women over 50 years of age.
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <5600 | 20 | 198 | 6,5 | 1-89-22,12 | 0,0008* |
| II | 5600-6100 | 3 | 192 | Ref. | Ref. | Ref. |
| III | >6100 | 22 | 399 | 3,5 | 1,04-11,94 | 0,029 |
*statistically significant result (p<0.05) P.437571
Evaluation of the cancer risk in women - BRCA1 carriers
Women with a mutation in the BRCA1 gene who do not smoke cigarettes have an almost 3-fold reduced risk of cancer if their zinc level is in the range of 6000-6700 µg/l. Such a correlation cannot be found among cigarette smokers.
Incidence of cancers depending on the concentration of zinc in the blood in women carriers of mutations in the BRCA1 gene who did not smoke cigarettes (selected ranges)
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 6000-6700 | 8 | 218 | Ref. | Ref. | Ref. |
| II | <6000&>6700 | 49 | 481 | 2,8 | 1,3-6,0 | 0,006* |
*statistically significant result (p<0.05) P.425603
Evaluation of the cancer risk in men
Men who have not smoked cigarettes have a nearly four-fold reduced risk of cancer if their blood zinc concentration is in the range of 5600-6350 µg/l.
Częstość występowania raków w zależności od stężenia cynku we krwi u mężczyzn niepalących (wybrane zakresy)
| Quartiles | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 5600-6350 | 5 | 318 | Ref. | Ref. | Ref. |
| II | <5600&>6350 | 41 | 687 | 3,8 | 1,5-9,7 | 0,0018* |
*statistically significant result (p<0.05) P.437894
Survival of patients with breast cancer
Women diagnosed with breast cancer and serum zinc concentration > 1000 µg/l have a nearly 6-fold reduced risk of death compared to women with zinc concentration < 700 µg/l.
The table shows the incidence of death according to serum zinc concentration in women with breast cancer (n=538).
Incidence of death depending on serum zinc concentration in women with breast cancer within 10 years of diagnosis (selected ranges)
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | ≤700 | 33 | 25 | 5,68 | 2,09-15,42 | 0,0003* |
| II | 700-850 | 181 | 54 | 2,24 | 0,91-5,53 | 0,088 |
| III | 851-1000 | 158 | 36 | 1,71 | 0,68-4,31 | 0,30 |
| IV | ≥1000 | 45 | 6 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05) P.434767
Survival of patients with prostate cancer
Men diagnosed with prostate cancer whose serum zinc concentration is in the range of 1000-1200 µg/l have an almost 4-fold reduced risk of death compared to men whose serum zinc concentration is < 900 µg/l.
Incidence of death depending on serum zinc concentration in men with prostate cancer within 5 years of diagnosis (quarters)
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | ≤760,38 | 56 | 34 | 8,4 | 3,31-21,33 | <0,0001* |
| II | 760,39-839,63 | 75 | 14 | 2,58 | 0,94-7,06 | 0,095 |
| III | 839,64-931,72 | 78 | 11 | 1,95 | 0,69-5,53 | 0,31 |
| IV | ≥931,73 | 83 | 6 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
Among men diagnosed with prostate cancer whose serum zinc concentration is in the range of 1000-1200, a nearly 4-fold reduced risk of death was observed compared to men whose zinc concentration is lower than 900 µg/l.
Incidence of death depending on serum zinc concentration in men with prostate cancer within 5 years of diagnosis (selected ranges)
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | <900 | 180 | 54 | 3,7 | 1,09-12,48 | 0,03* |
| II | 1000-1200 | 37 | 3 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The above results are the subject of patent application P.437046.
Survival of patients with laryngeal cancer
Among people with laryngeal cancer, the risk of death was more than twice reduced in patients with zinc concentrations above 688 µg/l in serum compared to those with concentrations <580 µg/l.
Frequency of deaths depending on serum zinc concentration in patients with laryngeal cancer within 5 years of diagnosis (tertiles)
| Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
| I | 357,76-580,38 | 52 | 52 | 2,45 | 1,4-4,4 | <0,01* |
| II | 584,46-688,89 | 67 | 37 | 1,35 | 0,8-2,4 | 0,31 |
| III | 688,94-1317,87 | 76 | 31 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05) P.427370
The above results are the subject of the publication Lubiński J. et al., Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes, Biomolecules2021, 11, 865.[40]
Copper performs various functions in the structure of proteins and as a catalyst thanks to the ability to change the degree of oxidation and reduction and occurs in the oxidized (Cu2+) or reduced (Cu+) state. Copper ions can participate in a wide range of interactions with proteins, enabling the formation of complex structures and mediating complex biochemical reactions.[41,42] Copper, like other trace elements, is, on the one hand, essential for the body, but on the other hand, it is very dangerous. . In general, however, conditions that are characterized by general or cell-specific copper accumulation are rare and most often occur as a result of specific genetic disorders.[43]
Evaluation of the cancer risk in women - BRCA1 non-carriers
Non-smoking women under 50 with blood copper concentrations between 850-1000 µg/l have an almost three-fold reduced risk of cancer.
Incidence of cancer in non-smoking women under 50 years of age with no mutations in the BRCA1 gene (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 850-1000 | 16 | 493 | Ref. | Ref. | Ref. |
| II | <850 | 13 | 156 | 2,6 | 1,2-5,5 | 0,02* |
*statistically significant result (p<0.05) P.438572
Environmental lead contamination is an ongoing problem for developing societies. The toxic effect of lead mainly concerns its effect on the hematopoietic system[44], the peripheral and central nervous system[45] and the gastrointestinal tract[46]. Due to the ubiquity of lead, virtually every person is exposed to contact with this element. Lead toxicity leads, among other things, to changes in the activity of many enzymes and disturbances in the functions of free and structural proteins in the cell[47]. Many studies suggest that an important molecular mechanism of lead toxicity is its participation in the formation of free oxygen radicals, which play a large role in the formation of intracellular damage and in the pathogenesis of many diseases, including malignant tumors[48]. According to the IARC classification of carcinogens, lead and its compounds belong to groups 2a and 2b, i.e. potentially carcinogenic [12].
Evaluation of the cancer risk in women - BRCA1 non-carriers
Women with blood lead concentration ≤ 8 µg/l have a significantly reduced risk of developing cancer by almost 9 times.
Cancer incidence depends on lead concentration in women. (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | ≤8 | 1 | 372 | Ref. | Ref. | Ref. |
| II | >8 | 26 | 1094 | 8,84 | 1,2-65,41 | 0,006* |
*statistically significant result (p<0.05) P.433150
Evaluation of the cancer risk in women - BRCA1 carriers
Women with blood lead concentration ≤ 8 µg/l have a significantly reduced risk of developing cancer by three times.
Cancer incidence depends on lead concentration in women. (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | ≤8 | 8 | 217 | Ref. | Ref. | Ref. |
| II | >8 | 87 | 812 | 2,9 | 1,4-6,1 | 0,002* |
*statistically significant result (p<0.05) P.433150
Evaluation of the cancer risk in men
Men under 60 years of age and non-smokers have an almost 8-fold reduced risk of developing the disease if their lead concentration is lower than 13.5 µg/l.
Cancer incidence depends on lead concentration in non-smoking men under 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <13,5 | 1 | 217 | Ref. | Ref. | Ref. |
| II | >13,5 | 16 | 447 | 7,8 | 1,02-59,0 | 0,017* |
*statistically significant result (p<0.05) P.437899
Men over 60 years of age, non-smokers have a four-fold reduced risk of cancer if their lead concentration is in the range of 20-50 µg/l in whole blood.
Cancer incidence depends on lead concentration in non-smoking men over 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 20-50 | 3 | 74 | Ref. | Ref. | Ref. |
| II | <20 & >50 | 15 | 90 | 4,1 | 1,1-14,8 | 0,02* |
*statistically significant result (p<0.05) P.437899
Manganese (Mn) is an essential nutrient involved in properly functioning the immune system, regulation of blood sugar levels and cellular energy, reproduction, digestion, bone growth, blood clotting and homeostasis, and defense against reactive oxygen species. The functions performed by manganese metalloproteins include oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. [49] Mn acts as a cofactor for various enzymes, including arginase, glutamine synthetase (GS), pyruvate carboxylase, and Mn superoxide dismutase (Mn-SOD) [50]. Mn tends to accumulate in the liver, pancreas, bones, and brain [50].
The literature contains studies describing the correlation between serum Mn concentration and the risk of breast and colorectal cancer. However, the published studies are retrospective, which makes them unreliable for the conclusion that manganese is a marker of cancer risk. In our Center, we assessed the correlation between manganese concentration and survival of women with breast cancer.
Survival of patients with breast cancer
Women diagnosed with breast cancer, whose serum manganese concentration is in the range of 1-10 µg/l, have a significantly reduced risk of death by over 1.5 times compared to other women.
The incidence of death depends on serum manganese concentration in women with breast cancer within 10 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | 1,0-10,0 | 197 | 49 | Ref. | Ref. | Ref. |
| II | <1 & > 10 | 204 | 87 | 1,55 | 1,09-2,20 | 0,01* |
*statistically significant result (p<0.05) P.438040
Chromium (Cr) is an element widely distributed in the Earth's crust. This element in small amounts is necessary for the proper functioning of the body, playing a role in the metabolic processes of glucose, some proteins, and fats [51–53]. Increased doses of chromium may have a strong toxic effect, destructively affecting the liver, kidneys, and hematopoietic system, and causing cancer [51,54,55]. Cr(VI) has been found to have a strong mutagenic effect. The results of association studies indicate a relationship between the concentration of chromium in the blood and the risk of cancer in the head, neck, and oral cavity.
In our Center, work was carried out on the correlation between serum chromium concentration and survival of women diagnosed with breast cancer.
Survival of patients with breast cancer
Women diagnosed with breast cancer whose serum chromium concentration is in the range of 0.20-3.5 µg/l have a significantly reduced risk of death by almost two times.
Incidence of death depending on serum chromium concentration in women with breast cancer within 10 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | 0,20-3,5 | 202 | 54 | Ref. | Ref. | Ref. |
| II | <0,20 & > 3,5 | 199 | 82 | 1,8 | 1,28-2,54 | 0,0002* |
*statistically significant result (p<0.05) P.438042
Women who smoke cigarettes and have been diagnosed with breast cancer have a significantly reduced risk of death by almost 4.5 times if their serum chromium concentration is higher than 0.5 µg/l.
Incidence of death depending on serum chromium concentration in smokers with breast cancer within 10 years of diagnosis (selected ranges).
| Group | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
| I | <0,5 | 157 | 56 | 4,46 | 1,39-14,26 | 0,01* |
| II | ≥0,5 | 38 | 3 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05) P.438042
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology 2020, 28, 667–695, doi:10.1007/s10787-020-00690-x.
- Combs, G.F.; Clark, L.C.; Turnbull, B.W. An Analysis of Cancer Prevention by Selenium. Biofactors 2001, 14, 153–159, doi:10.1002/biof.5520140120.
- Reddy, V.N.; Giblin, F.J.; Lin, L.R.; Dang, L.; Unakar, N.J.; Musch, D.C.; Boyle, D.L.; Takemoto, L.J.; Ho, Y.S.; Knoernschild, T.; et al. Glutathione Peroxidase-1 Deficiency Leads to Increased Nuclear Light Scattering, Membrane Damage, and Cataract Formation in Gene-Knockout Mice. Invest Ophthalmol Vis Sci 2001, 42, 3247–3255.
- Kuria, A.; Fang, X.; Li, M.; Han, H.; He, J.; Aaseth, J.O.; Cao, Y. Does Dietary Intake of Selenium Protect against Cancer? A Systematic Review and Meta-Analysis of Population-Based Prospective Studies. Crit Rev Food Sci Nutr 2020, 60, 684–694, doi:10.1080/10408398.2018.1548427.
- Jenkins, D.J.A.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Blanco Mejia, S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.C.; et al. Selenium, Antioxidants, Cardiovascular Disease, and All-Cause Mortality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Clin Nutr 2020, 112, 1642–1652, doi:10.1093/ajcn/nqaa245.
- Schomburg, L. The Other View: The Trace Element Selenium as a Micronutrient in Thyroid Disease, Diabetes, and Beyond. Hormones (Athens) 2020, 19, 15–24, doi:10.1007/s42000-019-00150-4.
- Kenfield, S.A.; Van Blarigan, E.L.; DuPre, N.; Stampfer, M.J.; L Giovannucci, E.; Chan, J.M. Selenium Supplementation and Prostate Cancer Mortality. J Natl Cancer Inst 2015, 107, 360, doi:10.1093/jnci/dju360.
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.; Falco, M.; et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients 2021, 13, 953, doi:10.3390/nu13030953.
- Pietrzak, S.; Wójcik, J.; Scott, R.J.; Kashyap, A.; Grodzki, T.; Baszuk, P.; Bielewicz, M.; Marciniak, W.; Wójcik, N.; Dębniak, T.; et al. Influence of the Selenium Level on Overall Survival in Lung Cancer. J Trace Elem Med Biol 2019, 56, 46–51, doi:10.1016/j.jtemb.2019.07.010.
- Lener, M.R.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer. Cancer Res Treat 2016, 48, 1056–1064, doi:10.4143/crt.2015.282.
- Rogoża-Janiszewska E. et al Serum Selenium Level and the 10-Year Survival after Melanoma. Biomedicines SI: Role of Trace Elements in Chemoprevention and Cancer Therapy 2021.
- List of Classifications – IARC Monographs on the Identification of Carcinogenic Hazards to Humans Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 20 July 2021).
- Marciniak, W.; Derkacz, R.; Muszyńska, M.; Baszuk, P.; Gronwald, J.; Huzarski, T.; Cybulski, C.; Jakubowska, A.; Falco, M.; Dębniak, T.; et al. Blood Arsenic Levels and the Risk of Familial Breast Cancer in Poland. Int J Cancer 2020, 146, 2721–2727, doi:10.1002/ijc.32595.
- Marciniak, W.; Matoušek, T.; Domchek, S.; Paradiso, A.; Patruno, M.; Irmejs, A.; Roderte, I.; Derkacz, R.; Baszuk, P.; Kuświk, M.; et al. Blood Arsenic Levels as a Marker of Breast Cancer Risk among BRCA1 Carriers. Cancers 2021, 13, 3345, doi:10.3390/cancers13133345.
- Fowler, B.A. Monitoring of Human Populations for Early Markers of Cadmium Toxicity: A Review. Toxicol Appl Pharmacol 2009, 238, 294–300, doi:10.1016/j.taap.2009.05.004.
- Vinceti, M.; Venturelli, M.; Sighinolfi, C.; Trerotoli, P.; Bonvicini, F.; Ferrari, A.; Bianchi, G.; Serio, G.; Bergomi, M.; Vivoli, G. Case-Control Study of Toenail Cadmium and Prostate Cancer Risk in Italy. Sci Total Environ 2007, 373, 77–81, doi:10.1016/j.scitotenv.2006.11.005.
- Qayyum, M.A.; Shah, M.H. Comparative Study of Trace Elements in Blood, Scalp Hair and Nails of Prostate Cancer Patients in Relation to Healthy Donors. Biol Trace Elem Res 2014, 162, 46–57, doi:10.1007/s12011-014-0123-4.
- Pirincci, N.; Gecit, I.; Gunes, M.; Kaba, M.; Tanik, S.; Yuksel, M.B.; Arslan, H.; Demir, H. Levels of Serum Trace Elements in Renal Cell Carcinoma Cases. Asian Pac J Cancer Prev 2013, 14, 499–502, doi:10.7314/apjcp.2013.14.1.499.
- Kellen, E.; Zeegers, M.P.; Hond, E.D.; Buntinx, F. Blood Cadmium May Be Associated with Bladder Carcinogenesis: The Belgian Case-Control Study on Bladder Cancer. Cancer Detect Prev 2007, 31, 77–82, doi:10.1016/j.cdp.2006.12.001.
- Wolf, C.; Strenziok, R.; Kyriakopoulos, A. Elevated Metallothionein-Bound Cadmium Concentrations in Urine from Bladder Carcinoma Patients, Investigated by Size Exclusion Chromatography-Inductively Coupled Plasma Mass Spectrometry. Anal Chim Acta 2009, 631, 218–222, doi:10.1016/j.aca.2008.10.035.
- Farzin, L.; Moassesi, M.E.; Sajadi, F.; Ahmadi Faghih, M.A. Evaluation of Trace Elements in Pancreatic Cancer Patients in Iran. Middle East Journal of Cancer 2013, 4, 79–86.
- Amaral, A.F.S.; Porta, M.; Silverman, D.T.; Milne, R.L.; Kogevinas, M.; Rothman, N.; Cantor, K.P.; Jackson, B.P.; Pumarega, J.A.; López, T.; et al. Pancreatic Cancer Risk and Levels of Trace Elements. Gut 2012, 61, 1583–1588, doi:10.1136/gutjnl-2011-301086.
- Wu, H.-D.I.; Chou, S.-Y.; Chen, D.-R.; Kuo, H.-W. Differentiation of Serum Levels of Trace Elements in Normal and Malignant Breast Patients. Biol Trace Elem Res 2006, 113, 9–18, doi:10.1385/BTER:113:1:19.
- Deeb, M.; El-Sheredy, H.; Mohammed, A. The Role of Serum Trace Elements and Oxidative Stress in Egyptian Breast Cancer Patients. Advances in Breast Cancer Research 2016, 05, 37–47, doi:10.4236/abcr.2016.51004.
- Sabir, S.; Akash, M.S.H.; Fiayyaz, F.; Saleem, U.; Mehmood, M.H.; Rehman, K. Role of Cadmium and Arsenic as Endocrine Disruptors in the Metabolism of Carbohydrates: Inserting the Association into Perspectives. Biomed Pharmacother 2019, 114, 108802, doi:10.1016/j.biopha.2019.108802.
- Lee, J.-E.; Kim, H.-R.; Lee, M.; Kim, N.-H.; Wang, K.-M.; Lee, S.; Park, O.; Hong, E.-J.; Youn, J.-W.; Kim, Y.-Y. Smoking-Related DNA Methylation Is Differentially Associated with Cadmium Concentration in Blood. Biochem Genet 2020, 58, 617–630, doi:10.1007/s10528-020-09965-y.
- Bolam, T.; Bersuder, P.; Burden, R.; Shears, G.; Morris, S.; Warford, L.; Thomas, B.; Nelson, P. Cadmium Levels in Food Containing Crab Brown Meat: A Brief Survey from UK Retailers. Journal of Food Composition and Analysis 2016, 54, 63–69, doi:10.1016/j.jfca.2016.10.005.
- Derkacz R. et al. Blood Cadmium Level and the Risk of Cancer in Women with BRCA1 Mutations. Cancers, SI: Advances in Inherited Breast and Ovarian Cancer and Its Imaging 2021.
- Nasiadek, M.; Stragierowicz, J.; Klimczak, M.; Kilanowicz, A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients 2020, 12, 2464, doi:10.3390/nu12082464.
- Puzanowska-Tarasiewicz, H.; Kuźmicka, L.; Tarasiewicz, M. Funkcje biologiczne wybranych pierwiastków i ich związków. 3, 3,. Polski Merkuriusz Lekarski : organ Polskiego Towarzystwa Lekarskiego. 2009, 419–422.
- To, P.K.; Do, M.H.; Cho, J.-H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int J Mol Sci 2020, 21, E2991, doi:10.3390/ijms21082991.
- Zaichick VYe, null; Sviridova, T.V.; Zaichick, S.V. Zinc in the Human Prostate Gland: Normal, Hyperplastic and Cancerous. Int Urol Nephrol 1997, 29, 565–574, doi:10.1007/BF02552202.
- Siddiqui, M.K.J.; Jyoti, null; Singh, S.; Mehrotra, P.K.; Singh, K.; Sarangi, R. Comparison of Some Trace Elements Concentration in Blood, Tumor Free Breast and Tumor Tissues of Women with Benign and Malignant Breast Lesions: An Indian Study. Environ Int 2006, 32, 630–637, doi:10.1016/j.envint.2006.02.002.
- Pasha, Q.; Malik, S.A.; Shah, M.H. Statistical Analysis of Trace Metals in the Plasma of Cancer Patients versus Controls. J Hazard Mater 2008, 153, 1215–1221, doi:10.1016/j.jhazmat.2007.09.115.
- el-Ahmady, O.; el-Maraghy, A.; Ibrahim, A.; Ramzy, S. Serum Copper, Zinc, and Iron in Patients with Malignant and Benign Pulmonary Diseases. Nutrition 1995, 11, 498–501.
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol Trace Elem Res 2002, 89, 1–11, doi:10.1385/BTER:89:1:1.
- Zhou, W.; Park, S.; Liu, G.; Miller, D.P.; Wang, L.I.; Pothier, L.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Christiani, D.C. Dietary Iron, Zinc, and Calcium and the Risk of Lung Cancer. Epidemiology 2005, 16, 772–779, doi:10.1097/01.ede.0000181311.11585.59.
- Zhang, X.; Giovannucci, E.L.; Smith-Warner, S.A.; Wu, K.; Fuchs, C.S.; Pollak, M.; Willett, W.C.; Ma, J. A Prospective Study of Intakes of Zinc and Heme Iron and Colorectal Cancer Risk in Men and Women. Cancer Causes Control 2011, 22, 1627–1637, doi:10.1007/s10552-011-9839-z.
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc Supplement Use and Risk of Prostate Cancer. J Natl Cancer Inst 2003, 95, 1004–1007, doi:10.1093/jnci/95.13.1004.
- Lubiński, J.; Jaworowska, E.; Derkacz, R.; Marciniak, W.; Białkowska, K.; Baszuk, P.; Scott, R.J.; Lubiński, J.A. Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes. Biomolecules 2021, 11, 865, doi:10.3390/biom11060865.
- Festa, R.A.; Thiele, D.J. Copper: An Essential Metal in Biology. Curr Biol 2011, 21, R877-883, doi:10.1016/j.cub.2011.09.040.
- Ceramella, J.; Mariconda, A.; Iacopetta, D.; Saturnino, C.; Barbarossa, A.; Caruso, A.; Rosano, C.; Sinicropi, M.S.; Longo, P. From Coins to Cancer Therapy: Gold, Silver and Copper Complexes Targeting Human Topoisomerases. Bioorg Med Chem Lett 2020, 30, 126905, doi:10.1016/j.bmcl.2019.126905.
- Linder, M.C. The Relationship of Copper to DNA Damage and Damage Prevention in Humans. Mutat Res 2012, 733, 83–91, doi:10.1016/j.mrfmmm.2012.03.010.
- Johnson, F.M. The Genetic Effects of Environmental Lead. Mutat Res 1998, 410, 123–140, doi:10.1016/s1383-5742(97)00032-x.
- Marchetti, C. Molecular Targets of Lead in Brain Neurotoxicity. Neurotox Res 2003, 5, 221–236, doi:10.1007/BF03033142.
- Tomczyk, J.; Lewczuk, E.; Abdrzejak, R. Ostre Zatrucia Organicznymi Związkammi Ołowiu.; Medycyna Pracy, 1999; Vol. 50;.
- Cellular Mechanisms of Lead Neurotoxicity – PubMed Available online: https://pubmed.ncbi.nlm.nih.gov/16501435/ (accessed on 20 July 2021).
- Nersesyan, A.; Kundi, M.; Waldherr, M.; Setayesh, T.; Mišík, M.; Wultsch, G.; Filipic, M.; Mazzaron Barcelos, G.R.; Knasmueller, S. Results of Micronucleus Assays with Individuals Who Are Occupationally and Environmentally Exposed to Mercury, Lead and Cadmium. Mutat Res 2016, 770, 119–139, doi:10.1016/j.mrrev.2016.04.002.
- Aschner, M.; Erikson, K. Manganese. Adv Nutr 2017, 8, 520–521, doi:10.3945/an.117.015305.
- Chen, P. Manganese Metabolism in Humans. Front Biosci 2018, 23, 1655–1679, doi:10.2741/4665.
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and Oxidative Mechanisms of Different Forms of Chromium. Toxicology 2002, 180, 5–22, doi:10.1016/s0300-483x(02)00378-5.
- Cefalu, W.T.; Hu, F.B. Role of Chromium in Human Health and in Diabetes. Diabetes Care 2004, 27, 2741–2751, doi:10.2337/diacare.27.11.2741.
- Vincent, J.B. The Biochemistry of Chromium. J Nutr 2000, 130, 715–718, doi:10.1093/jn/130.4.715.
- Anderson, R.A. Chromium Metabolism and Its Role in Disease Processes in Man. Clin Physiol Biochem 1986, 4, 31–41.
- Anderson, R.A. Chromium as an Essential Nutrient for Humans. Regul Toxicol Pharmacol 1997, 26, S35-41, doi:10.1006/rtph.1997.1136.
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum Selenium Levels Predict Survival after Breast Cancer. Breast Cancer Res Treat 2018, 167, 591–598, doi:10.1007/s10549-017-4525-9.
- Lubiński, J.; Marciniak, W.; Muszynska, M.; Jaworowska, E.; Sulikowski, M.; Jakubowska, A.; Kaczmarek, K.; Sukiennicki, G.; Falco, M.; Baszuk, P.; et al. Serum Selenium Levels and the Risk of Progression of Laryngeal Cancer. PLoS One 2018, 13, e0184873, doi:10.1371/journal.pone.0184873.
Home » Research
Research
Research projects
Prevention of Hereditary Breast Cancer through Personalized Optimization of Se, Zn, Fe Levels in the Body Using Dietary Supplements
Agreement No. INNOMED/I/16/NCBR/2014
Project Duration as per the Agreement: 01.10.2014-30.09.2019. The project co-financed by the European Union under the European Regional Development Fund
The project partner is the West Pomeranian University of Technology in Szczecin
Characteristics of the studied groups
The study groups, whose analyses form the basis for the results described below, were created from individuals registered in the Hereditary Cancer Center in Szczecin between 2009 and 2020. Each patient provided informed consent for the storage and use of biological material for scientific purposes. Blood and serum samples were collected between 8 AM and 2 PM, and patients fasted for at least 4 hours before collection. For the majority of patients, a sample was collected only once, but in some cases, multiple samples were taken during subsequent visits. Biological material was stored at -80°C until the element concentrations were determined. Each participant in the study completed a health and lifestyle questionnaire.
A total of 2962 healthy women were included in the prospective cohort. During 42 months of follow-up, 148 women were diagnosed with cancer.
The characteristics of the group are presented in the table.
| Cases (n=148) | Healthy (n=2814) |
Average age (range) | 56,46 (35-82) | 53 (33-84) |
Smoking current former never |
40 (27,03%) 34 (22,97%) 74 (50 %) |
605 (21,50%) 750 (26,65%) 1459 (51,84%) |
Hormone replacement therapy / oral contraceptives no yes missing data |
93 (62,84%) 54 (36,49%) 1 (0,67%) |
1467 (52,13%) 1316 (46,77%) 31 (1,1%) |
Adnexektomia no yes missing data |
135 (91,22%) 9 (6,08%) 4 (2,7%) |
2631 (93,50%) 175 (6,22%) 8 (0,28%) |
Cancers (location) breast uterus colon melanoma thyroid ovary cervix lymphoma bladder lung kidney stomach leukemia myeloma glioblastoma pancreas |
76 (51,4%) 12 (8,1%) 10 (6,8%) 7 (4,7%) 7 (7,73%) 6 (4,05%) 5 (3,38%) 5 (3,38%) 4 (2,7%) 4 (2,7%) 3 (2,03%) 3 (2,03%) 2 (1,35%) 2 (1,35%) 1 (0,68%) 1 (0,68%) |
– – – – – – – – – – – – – – – – |
Do kohorty prospektywnej włączono 1324 zdrowe (bez zdiagnozowanego nowotworu złośliwego) kobiety, u których wykryto mutację w genie BRCA1. Uczestniczki zostały poddane średnio 40 miesięcznej obserwacji, w trakcie której u 107 kobiet zdiagnozowano nowotwór złośliwy.
The characteristics of the group are presented in the table.
| Cases (n=107) | Healthy (n=1217) |
Average age (range) | 44,25 (26-66) | 40,5 (25-69) |
Smoking current former never missing data |
24 (22,43%) 27 (25,23%) 55 (51,4%) 1 (0,93%) |
254 (20,87%) 249 (20,46%) 699 (57,44%) 15 (1,23%) |
Hormone replacement therapy / oral contraceptives no yes missing data |
46 (43%) 60 (56,07%) 1 (0,93%) |
603 (49,55%) 592 (48,64%) 22 (1,81%) |
Adnexektomia no yes missing data |
52 (48,6%) 55 (51,4%) 0 |
688 (56,53%) 510 (41,92%) 19 (1,56%) |
Cancers (location) breast ovary cervix peritoneum stomach colon leukemia pancreas skin larynx thyroid bladder |
80 (74,8%) 15 (14%) 3 (2,8%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) 1 (0,9%) |
– – – – – – – – – – – – |
Do kohorty prospektywnej włączono zdrowych (bez zdiagnozowanego nowotworu złośliwego) 2956 mężczyzn. Zostali oni poddani średnio 76 miesięcznej obserwacji, w trakcie której u 144 mężczyzn zdiagnozowano nowotwór złośliwy. Każdy z uczestników badania wypełnił ankietę o stanie zdrowia oraz stylu życia.
Charakterystykę grupy prospektywnej przedstawiono w poniższej tabeli.
| Cases (n=144) | Healthy (n=2812) |
Average age (range) | 60,5 (36-76) | 52 (31-87) |
Smoking current former never |
42 (29,17%) 55 (38,19%) 47 (32,64 %) |
829 (29,48%) 975 (34,67%) 1008 (35,85%) |
Cancers (location) prostate skin kidney colon bladder blood lung liver thyroid pancreas stomach breast esophagus pituitary gland salivary glands testis |
58 (40,28%) 16 (11,11%) 13 (9,03%) 13 (9,03%) 12 (8,33%) 9 (6,25%) 6 (4,17%) 4 (2,78%) 4 (2,78%) 2 (1,39%) 2 (1,39%) 1 (0,69%) 1 (0,69%) 1 (0,69%) 1 (0,69%) 1 (0,69%) |
– – – – – – – – – – – – – – – – |
Do badań włączono 538 pacjentek ze zdiagnozowanym i potwierdzonym histopatologicznie rakiem piersi. Krew została zebrana w latach 2009-2015 w momencie diagnozy raka piersi, przed rozpoczęciem leczenia. W 2020 roku zebrano informację o zgonach w trakcie obserwacji prospektywnej. Uzyskano ją z Departamentu Ewidencji Państwowych – Ministerstwa Cyfryzacji. Średni okres obserwacji dla pacjentek żyjących wyniósł 9 lat (zakres 5-12 lat).
Charakterystyka grupy została przedstawiona w poniższej tabeli.
| Alive (n=417) | Deaths (n=121) |
Average age (range) | 55,87 (25-85) | 61,07 (28-91) |
Smoking current former never missing data |
89 (21,3%) 112 (26,9%) 206 (49,4%) 10 (2,4%) |
26 (21,5%) 27 (22,3%) 65 (53,7%) 3 (2,5%) |
Estrogen receptor positive (ER+) negative (ER-) missing data |
287 (68,8%) 114 (27,3%) 16 (3,8%) |
79 (65,3%) 32 (26,4%) 10 (8,26%) |
BRCA1 mutation | 50 (12%) | 11 (9,1%) |
Chemotherapy yes no missing data |
211 (50,6%) 174 (41,7%) 32 (7,7%) |
65 (53,7%) 36 (29,8%) 20 (16,5%) |
Radiotherapy yes no missing data |
244 (58,5%) 122 (29,3%) 51 (12,2%) |
56 (46,3%) 35 (28,9%) 30 (24,8%) |
Tamoxifen yes no missing data |
282 (67,6%) 122 (29,3%) 13 (3,1%) |
79 (65,3%) 33 (27,3%) 9 (7,4%) |
Type of operation mastectomy lumpectomy missing data |
261 (62,6%) 131 (31,4%) 25 (6%) |
82 (67,8%) 17 (14%) 22 18,2%) |
The study included 357 patients with diagnosed and histopathologically confirmed prostate cancer. Blood was collected between 2009 and 2015 at the time of diagnosis of prostate cancer before treatment. The average follow-up period for patients was 5 years.
| Alive (n=293) | Deaths (n=102) |
Gleason scale 6 7 8 9 10 missing data |
85 (29%) 164 (56%) 20 (6,8%) 17 (5,8%) 2 (0,7%) 5 (1,7%) |
39 (38,2%) 35 (34,3%) 9 (8,8%) 11 (10,8%) 7 (6,9%) 2 (1,96%) |
Clinical stage 1-2 3 4 missing data |
7 (2,7%) 232 (79,2%) 51 (17,4%) 2 (0,7%) |
4 (3,9%) 45 (44,1%) 53 (52%) – |
Radiotherapy yes no |
84 (28,7%) 209 (71,3%) |
24 (23,5%) 78 (76,5%) |
Chemotherapy yes no |
3 (1%) 290 (99%) |
4 (3,9%) 98 (96,1%) |
Hormonetherapy yes no |
59 (20,1%) 234 (79,9%) |
40 (39,2%) 62 (60,8%) |
Prostatectomy yes no |
233 (79,5%) 60 (20,5%) |
33 (32,4%) 69 (67,6%) |
Orchidectomy yes no |
8 (2,7%) 285 (97,3%) |
11 (10,8%) 91 89,2%) |
The group included 315 patients treated surgically between July 2009 and February 2017 due to squamous cell carcinoma of the larynx. The patients constituted over 70% of all patients operated on at the Otolaryngology Clinic of the Pomeranian Medical University in Szczecin. Clinical information was collected from all patients regarding age at onset, gender, clinical stage, radiotherapy, chemotherapy and smoking intensity (pack-years).
| n=315 | % |
Average age of cancer diagnosis (range) | 61,1 (41-86) |
|
Sex women men |
49 266 |
15,6 84,4 |
Clinical stage 1 2 3 4 |
72 42 70 131 |
22,8 13,3 22,2 41,6 |
Pack-years (average, range) | 37,11 (0-150) |
|
Treatment radiotherapy chemotherapy |
142 32 |
45,1 10,1 |
The study group consisted of 302 patients diagnosed with lung cancer. The patients came from the Department of Thoracic Surgery in Szczecin-Zdunowo. Patients were hospitalized between February 2010 and December 2011. Blood was collected at the time of diagnosis, before the start of treatment.
|
| % |
Sex
Men Women |
196 106 |
64,9 35,1 |
Average age (range) | 64,2 (43-86) | – |
Pack-years (range) | 33,2 (0-232,8) | – |
Smoking
yes no |
283 19 |
93,7 6,3 |
Clinical stage 1 2 3 4 |
129 75 79 19 |
42,7 24,8 26,2 6,3 |
Radiotherapy
yes no |
78 224 |
25,8 74,2 |
Chemotherapy
yes no |
105 197 |
34,8 65,2 |
Histological type
Adenocarcinoma Squamous cell carcinoma Large cell cancer Large and Small Cell Mixed Carcinoma Small Cell Carcinoma Other |
136 124 21 5 3 13 |
45 41,1 7 1,7 1 4,3 |
Do grupy badanej włączono 100 pacjentów z rakiem trzustki.
Charakterystyka grupy została przedstawiona w poniższej tabeli.
| New cancer diagnosis |
Year of birth (range) | 1930-1976 |
Average age at the blood donation (range) | 63,4 (35-84) |
Sex
men women |
59 41 |
First-Degree Relatives
with pancreatic cancer with other cancer |
4 43 |
Smoking
yes no |
27 73 |
Pack-years (range) | 28,99 (2-50) |
Do grupy włączono 375 pacjentów ze zdiagnozowanym czerniakiem złośliwym zdiagnozowanych w latach 2006-2016. Krew pacjentów została zabezpieczona w momencie diagnozy, przed rozpoczęciem leczenia.
Charakterystyka grupy badanej została przedstawiona w poniższej tabeli.
| Alive (n=344) | Deaths (n=31) |
Average age (range) | 54,6 (21-90) | 64,3 (38-86) |
Sex women men |
218 (63%) 126 (37%) |
14 (45%) 17 (55%) |
Clinical stage 2 3 4-5 |
70 (20%) 145 (42%) 129 (38%) |
1 (3,2%) 12 (39%) 18 (58%) |
Research results
W organizmie selen działa poprzez białka, do których jest wbudowany w postaci selenocysteiny. Jako składnik selenobiałek selen odgrywa rolę enzymatyczną, jak i strukturalną[1]. Do jednych z ważniejszych funkcji selenobiałek należy udział w produkcji hormonów tarczycy, pobudzanie układu immunologicznego, oraz ochrona przed stresem oksydacyjnym.[2] Zarówno niedobór jak i nadmiar tego pierwiastka może mieć niekorzystny wpływ na organizm. Prowadzi to do m.in. do zaburzeń pracy serca, zwyrodnienia serca i wątroby, zwiększenia ryzyka choroby nadciśnieniowej, ograniczenia sprawności układu odpornościowego, zaburzenia funkcji tarczycy, zaburzenia mineralizacji kości i prawidłowego wykształcenia zębów oraz zwiększenia ryzyka chorób nowotworowych. [3–7]
Evaluation of the cancer risk in women - BRCA1 non-carriers
Women over 60 years old and non-smokers have a significantly 4.5-fold reduced risk if their blood concentration is in the range of 94-104 µg/l. However, women over 60 years of age who currently smoke or have smoked in the past have a significantly 3.5-fold reduced risk of cancer if their blood selenium concentration is > 110 µg/l.
Incidence of cancer in non-smoking women over 60 years of age (selected ranges).
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | 94-104 | 3 | 114 | Ref. | Ref. | Ref. |
II | <94 | 11 | 93 | 4,5 | 1,2-16,6 | 0,02* |
*statistically significant result (p<0.05) P.438038
Incidence of cancer in smoking women over 60 years of age (selected ranges).
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | >110 | 4 | 97 | Ref. | Ref. | Ref. |
II | <110 | 25 | 171 | 3,5 | 1,2-10,5 | 0,02* |
*wynik istotny statystycznie (p<0,05) P.438038
Evaluation of the cancer risk in women - BRCA1 carriers
Women under 50 years of age with blood selenium concentrations in the range of 70-80 µg/l tend to have a nearly 5-fold reduced risk of cancer compared to women with selenium concentrations outside the presented range.
Cancer incidence depending on blood selenium concentration among women under 50 years of age (selected ranges).
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | 70-80 | 1 | 56 | Ref. | Ref. | Ref. |
II | <70 & >80 | 75 | 904 | 4,6 | 0,6-34,1 | 0,1* |
*wynik nieistotny statystycznie (p>0,05) P.435603
Women over 50 years of age with blood selenium concentrations in the range of 95-120 µg/l have a more than twofold reduced risk of cancer compared to women with selenium concentrations outside the presented range.
Cancer incidence depending on blood selenium concentration among women over 50 years of age (selected ranges).
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | 95-120 | 13 | 156 | Ref. | Ref. | Ref. |
II | <95 & >120 | 20 | 102 | 2,3 | 1,1-4,9 | 0,03* |
*statistically significant result (p <0.05) P.435603
Evaluation of the cancer risk in men
Men who do not smoke cigarettes and have selenium concentrations in the blood range of 100-110 µg/l have a nearly 4-fold reduced risk of cancer compared to men with selenium concentrations outside this range.
Cancer incidence depending on blood selenium concentration among non-smoking men (selected ranges).
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | 100-110 | 5 | 260 | Ref. | Ref. | Ref. |
II | <95 | 20 | 291 | 3,6 | 1,3-9,7 | 0,008* |
*wynik istotny statystycznie (p <0,05) P.437898
However, men over 60 years of age and those who have smoked cigarettes in the past or currently have a nearly 11-fold reduced risk of developing cancer if the selenium concentration in the blood is >115 µg/l, compared to men with the selenium concentration in the blood <115 µg/ l.
Cancer incidence depends on blood selenium concentration among smoking men over 60 years of age (selected ranges).
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | <115 | 1 | 83 | Ref. | Ref. | Ref. |
II | ≥115 | 56 | 427 | 10,9 | 1,5-79,8 | 0,0013* |
*statistically significant result (p < 0.05) P.437898
The Kaplan-Meier curve for the above correlation is shown below.

-
-
Survival of patients with breast cancer
Women with breast cancer have a significantly greater chance of 10-year survival if their serum selenium concentration is >94.7 µg/l. Women with serum selenium concentration < 70 µg/l have a more than 25-fold increased risk of death compared to the subgroup with serum selenium concentration 95-102.5 µg/l.
Incidence of death depending on serum selenium concentration in women with breast cancer within 10 years of diagnosis (quarters).
Quartiles | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
I | 52,1-76,7 | 89 | 46 | 2,35 | 1,12-4,55 | 0,01* |
II | 76,8-85,1 | 105 | 29 | 1,52 | 0,76-3,02 | 0,23 |
III | 85,2-94,6 | 105 | 29 | 1,95 | 1,01-3,76 | 0,047* |
IV | 94,7-171,5 | 118 | 17 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The incidence of death depends on serum selenium concentration in women with breast cancer within 10 years of diagnosis (ranges).
Quartiles | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
I | < 70 | 32 | 28 | Ref. | Ref. | Ref. |
II | 95-102,5 | 62 | 2 | 27,1 | 6,7-121,2 | <0,0001* |
*statistically significant result (p<0.05)
The above results are part of the publication by Szwiec M et al., Serum Selenium Level Predicts 10-Years Survival after Breast Cancer; Nutrients 2021, 13(3), 953. [8]
Survival of patients with prostate cancer
Men with prostate cancer and serum selenium concentration in the range of 85-105 µg/l have a significantly reduced risk of death by over 8 times compared to the subgroup with serum selenium concentration below 70 µg/l.
The incidence of death depends on serum selenium concentration in men with prostate cancer within 5 years of diagnosis (selected ranges).
Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
I | ≤70 | 61 | 33 | 8,6 | 3,4-21,7 | <0,0001* |
II | 85-105 | 95 | 6 | Ref. | Ref. | Ref. |
*wynik istotny statystycznie (p <0,05) P.437046
Survival of patients with lung cancer
It has been observed that people whose serum selenium concentration is >67.4 µg/l have a greater chance of longer survival after a lung cancer diagnosis than people with a low serum selenium concentration (<55.1 µg/l).
Frequency of deaths depending on serum selenium concentration in patients with stage I lung cancer within 3 years of diagnosis (tertiles).
Tertiles | Range [µg/l] | total | OR | 95%CI | p |
I | 33,46-57,91 | 43 | 2,73 | 1,21-6,11 | 0,01* |
II | 57,92-68,86 | 42 | 1,88 | 0,83-4,28 | 0,13 |
III | 69,29-108,27 | 44 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The above results are part of the publication by Pietrzak S et al., Influence of the selenium level on overall survival in lung cancer, Journal of Trace Elements in Medicine and Biology 2019, 56:46-51.[9]
Survival of patients with pancreatic cancer
Patients diagnosed with pancreatic cancer have a 3-fold greater chance of 6-month survival if their serum selenium concentration is ≥ 63.67 µg/l compared to patients with selenium concentration below this value.
The incidence of death depends on serum selenium concentration in patients with pancreatic cancer within 6 months of diagnosis (selected ranges).
Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
I | < 63,67 | 24 | 33 | 0,4 | 0,1-0,97 | 0,03* |
II | ≥ 63,67 | 22 | 11 | Ref. | Ref. | Ref. |
The above results are part of the publication by Lener M et al., Serum concentration of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer, Cancer Res Treat., 2016, 48(3):1056-1064.[10]
Survival of patients with melanoma
Patients diagnosed with malignant melanoma have a significantly greater chance of 10-year survival if their serum selenium concentration is higher than 96 µg/l. Patients with serum selenium concentration 96 µg/l.
The incidence of death depends on serum selenium concentration in patients with malignant melanoma within 10 years of diagnosis (quarters).
Quartiles | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
I | 56,7-76,2 | 78 | 16 | 5,83 | 1,32-25,8 | 0,02* |
II | 76,4-85,01 | 86 | 7 | 3,37 | 0,7-16,3 | 0,13 |
III | 85,15-96,06 | 88 | 6 | 3,34 | 0,67-16,7 | 0,14 |
IV | 96,15-168 | 92 | 2 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05)
The above results are the subject of the publication by Rogoża-Janiszewska E. et al., Serum selenium level and the 10-year survival after melanoma, Biomedicines, 2021.[11]
Arsenic and its compounds are among the most recognized toxins. According to the classification of the International Agency for Research on Cancer (IARC), arsenic and its compounds have been categorized as Group 1[12] carcinogens, meaning they are definite human carcinogens. The clinical manifestations resulting from inhalation or ingestion of arsenic compounds vary widely. Depending on the concentration, duration of exposure, and route of absorption, the effects of arsenic interaction with tissues range from relatively benign, such as hypopigmentation, to life-threatening tumors (WHO). Based on existing literature, it can be concluded that not only high but also slightly elevated arsenic concentrations may be linked to cancers, especially in women.
Evaluation of the cancer risk in women - BRCA1 non-carriers
Women with an arsenic concentration in blood below 0.6 µg/l have a significant, almost 5-fold reduced risk of developing cancers, especially breast cancer, compared to women with an arsenic concentration above 0.6 µg/l (OR=4.7; p=0 .0004; 95%CI:1.9-11.7).
Incidence of breast cancer depending on arsenic concentration in blood (selected ranges)
Group | Range | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | <0,6 | 5 | 735 | Ref. | Ref. | Ref. |
II | 0,6-0,81 | 18 | 723 | 3,7 | 1,4-10 | 0,01* |
III | 0,82-1,25 | 21 | 719 | 4,3 | 1,6-11-5 | 0,002* |
IV | >1,25 | 32 | 709 | 6,6 | 2,6-17,1 | <0,0001* |
Selected ranges | ||||||
I | <0,6 | 5 | 735 | Ref. | Ref. | Ref. |
II | ≥0,6 | 71 | 2222 | 4,7 | 1,9-11,7 | 0,0004* |
*wynik istotny statystycznie (p <0,05) P.425602
The Kaplan-Meier curve for the above correlation is shown below.

-
-
Powyższe wyniki stanowią przedmiot publikacji Marciniak W. et al., Blood arsenic levels and the risk of familial breast cancer in Poland, Int J Cancer, 2020, 146 (10): 2721-2727.[13]
Evaluation of the cancer risk in women - BRCA1 carriers
Women with an arsenic concentration in blood below 0.85 µg/l have a significantly reduced risk of developing cancer by approximately two times compared to women with an arsenic concentration above 0.85 µg/l (OR=2.55; p=0.0006; 95% CI:1.47-4.43).
The incidence of cancer depends on the concentration of arsenic in the blood.
Group | Range | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | <0,85 | 18 | 513 | Ref. | Ref. | Ref. |
II | >0,85 | 49 | 548 | 2,55 | 1,47-4,43 | 0,0006* |
*wynik istotny statystycznie (p<0,05)
The above results are part of the publication by Marciniak W. et al., Blood Arsenic Levels as a Marker of Breast Cancer Risk among BRCA1 Carriers, Cancers 2021, 13(13), 3345. [14]
Evaluation of the cancer risk in men
Men whose blood arsenic concentration is between 0.7 and 1.14 µg/l have a nearly 5-fold reduced risk of cancer.
Cancer incidence in men depending on arsenic concentration in blood (selected ranges)
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | 0,7-1,14 | 28 | 788 | Ref. | Ref. | Ref. |
II | <0,7 & > 1,14 | 116 | 2003 | 4,8 | 1,1-2,5 | 0,03* |
*wynik istotny statystycznie (p<0,05) P.437896
Survival of patients with prostate cancer
Men with serum arsenic concentration in the range of 0.7-1.0 µg/l have a significantly reduced risk of death by more than 3 times compared to the subgroup with serum arsenic concentration < 0.7 µg/l.
The incidence of death depends on serum arsenic concentration in men with prostate cancer within 5 years of diagnosis (selected ranges).
Group | Range [µg/l] | Deaths | Alive | OR | 95%CI | p |
I | <0,7 | 22 | 50 | 3,18 | 1,63-6,22 | 0,0009* |
II | 0,7-1,0 | 22 | 159 | Ref. | Ref. | Ref. |
III | >1,0 | 21 | 83 | 1,83 | 0,95-3,52 | 0,085 |
*wynik istotny statystycznie (p<0,05) P.437046
According to the classification of the International Agency for Research on Cancer (IARC), cadmium and its compounds have been categorized as Group 1 [12] human carcinogens. The adverse effects of cadmium and its compounds can lead to kidney diseases, cardiovascular diseases, hypertension, anemia, liver damage, disorders of the reproductive organs, immune system disorders, deficiencies of iron, copper, and zinc, as well as the development of cancer. Numerous studies describe increased levels of cadmium in the biological material of individuals who have developed malignant tumors of the prostate, kidney, bladder, pancreas, and breast.
The literature mentions three main sources of cadmium: diet, smoking, and occupational exposure[25]. The concentration of cadmium in food products is strongly dependent on the content of this element in the environment - air, soil, and water[25]. The concentration of cadmium in the blood is strongly correlated with smoking tobacco products. In non-smokers, Cd concentration is lower compared to smokers[26]. The occupational group most exposed to cadmium are workers in the zinc, steel, and copper industries, as well as in the production of nickel-cadmium batteries, solar cells, and jewelry[27].
Evaluation of the cancer risk in women - BRCA1 non-carriers
The table below shows the distribution of subjects in our chosen range. The risk of cancer is reduced more than 8 times among women whose concentration is in the range of 0.28-0.33 µg/l.
Cancer incidence depending on blood cadmium concentration in non-smoking women over 50 years of age (selected range)
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 0,28-0,33 | 1 | 126 | Ref. | Ref. | Ref. |
| II | <0,28 & >0,33 | 44 | 665 | 8,34 | 1,1-61,1 | 0,009* |
*wynik istotny statystycznie (p <0,05) P.437608
Evaluation of the cancer risk in women - BRCA1 carriers
In women under 51 years of age with blood cadmium concentration ≤0.32 µg/l, the risk is reduced by more than 8 times compared to women with higher cadmium levels.
Cancer incidence depending on blood cadmium concentration in women under 51 years of age (selected range)
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | ≤0,32 | 1 | 92 | Ref. | Ref. | Ref. |
| II | >0,32 | 22 | 244 | 8,30 | 1,1-62,46 | 0,013* |
*wynik istotny statystycznie (p<0,05) PAT.237085
The above results are the subject of a publication sent for publication by Derkacz R. et al., Blood Cadmium Level and the Risk of Cancer in Women with BRCA1 Mutations, Cancers, 2021.[28]
Evaluation of the cancer risk in men
Men who have never smoked cigarettes and whose blood cadmium concentration is below 0.14 µg/l have a nearly 6-fold reduced risk of cancer compared to men with a blood cadmium concentration above 0.28 µg/l.
Cancer incidence depending on blood cadmium concentration in non-smoking men (quarters)
| Quartiles | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 0,03-0,14 | 4 | 260 | Ref. | Ref. | Ref. |
| II | 0,14-0,21 | 7 | 257 | 1,77 | 0,51-6,12 | 0,54 |
| III | 0,21-0,28 | 15 | 249 | 3,92 | 1,28-11,96 | 0,02 |
| IV | 0,28-2,34 | 21 | 242 | 5,64 | 1,91-16,67 | 0,0004* |
*statistically significant result (p<0.05) P.437897
Zinc is an essential element for the proper functioning of the body. It plays a protective role against free radicals, including being part of superoxide dismutase (SOD2). It is also involved in immune processes, contributes to the proper functioning of the skin and mucous membranes, and is involved in storing and secreting insulin from the pancreas. Zinc maintains the ion balance of other trace elements, including selenium, magnesium, and copper, and also plays a detoxifying role with heavy metals. Deficiency of this element leads to serious disorders such as immunodeficiencies, inflammation (including SARS-CoV-2), impaired wound healing, reduced fertility, and vision problems [29,30].
It has been observed that zinc levels change in cancer cells[31]. Normal prostate epithelial cells accumulate zinc, while in cancer cells the level of this element is significantly reduced.[32] Zinc is believed to have anti-cancer effects by inhibiting the growth of cancer cells and activating apoptosis. There are known studies assessing the relationship between zinc concentration and the risk of cancer, but their results are divergent. Some of these studies say that serum zinc concentration is higher in people with cancer [33-35], while others say that the level is lower.[36] The results of research conducted so far also suggest that an appropriate amount of zinc in the diet has a chemopreventive effect. People whose diet is rich in zinc have a lower risk of lung cancer than people following a zinc-poor diet (OR 0.71; 95% CI 0.5-0.99). [37] The risk of colorectal cancer is also lower with a zinc-rich diet (RR 0.86; 95% CI 0.73-1.02).[38] However, zinc supplementation in very high doses above 100 mg/day (the recommended daily intake of zinc is 8 mg/day for women and 12 mg/day for men) has the opposite effect, significantly increasing the risk of prostate cancer (RR 2.29; 95% CI 1.06 – 4.95, p=0.03).[39] The results of research conducted in our Center are presented below.
Evaluation of the cancer risk in women - BRCA1 non-carriers
Non-smoking women over 50 years of age with a zinc concentration in the blood in the range of 5600-6100 µg/l have a 6.5-fold reduced risk of cancer compared to women with a zinc concentration below 5600 µg/l, and a 3.5-fold reduced risk compared to women with zinc concentration above 6100 µg/l.
Incidence of cancer depending on the concentration of zinc in the blood in non-smoking women over 50 years of age.
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | <5600 | 20 | 198 | 6,5 | 1-89-22,12 | 0,0008* |
II | 5600-6100 | 3 | 192 | Ref. | Ref. | Ref. |
III | >6100 | 22 | 399 | 3,5 | 1,04-11,94 | 0,029 |
*wynik istotny statystycznie (p<0,05) P.437571
Evaluation of the cancer risk in women - BRCA1 carriers
Kobiety z mutacją w genie BRCA1, które nie paliły papierosów mają prawie 3 krotnie obniżone ryzyko zachorowania na raka, jeśli ich poziom cynku znajduje się w zakresie 6000-6700 µg/l. Takiej korelacji nie można stwierdzić wśród osób palących papierosy.
Incidence of cancers depending on the concentration of zinc in the blood in women carriers of mutations in the BRCA1 gene who did not smoke cigarettes (selected ranges)
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | 6000-6700 | 8 | 218 | Ref. | Ref. | Ref. |
II | <6000&>6700 | 49 | 481 | 2,8 | 1,3-6,0 | 0,006* |
*wynik istotny statystycznie (p<0,05) P.425603
Evaluation of the cancer risk in men
Men who have not smoked cigarettes have a nearly four-fold reduced risk of cancer if their blood zinc concentration is in the range of 5600-6350 µg/l.
Częstość występowania raków w zależności od stężenia cynku we krwi u mężczyzn niepalących (wybrane zakresy)
Quartiles | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | 5600-6350 | 5 | 318 | Ref. | Ref. | Ref. |
II | <5600&>6350 | 41 | 687 | 3,8 | 1,5-9,7 | 0,0018* |
*wynik istotny statystycznie (p<0,05) P.437894
Survival of patients with breast cancer
Women diagnosed with breast cancer and serum zinc concentration > 1000 µg/l have a nearly 6-fold reduced risk of death compared to women with zinc concentration < 700 µg/l.
The table shows the incidence of death according to serum zinc concentration in women with breast cancer (n=538).
Incidence of death depending on serum zinc concentration in women with breast cancer within 10 years of diagnosis (selected ranges)
Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
I | ≤700 | 33 | 25 | 5,68 | 2,09-15,42 | 0,0003* |
II | 700-850 | 181 | 54 | 2,24 | 0,91-5,53 | 0,088 |
III | 851-1000 | 158 | 36 | 1,71 | 0,68-4,31 | 0,30 |
IV | ≥1000 | 45 | 6 | Ref. | Ref. | Ref. |
*wynik istotny statystycznie (p<0,05) P.434767
Survival of patients with prostate cancer
Men diagnosed with prostate cancer whose serum zinc concentration is in the range of 1000-1200 µg/l have an almost 4-fold reduced risk of death compared to men whose serum zinc concentration is < 900 µg/l.
Incidence of death depending on serum zinc concentration in men with prostate cancer within 5 years of diagnosis (quarters)
Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
I | ≤760,38 | 56 | 34 | 8,4 | 3,31-21,33 | <0,0001* |
II | 760,39-839,63 | 75 | 14 | 2,58 | 0,94-7,06 | 0,095 |
III | 839,64-931,72 | 78 | 11 | 1,95 | 0,69-5,53 | 0,31 |
IV | ≥931,73 | 83 | 6 | Ref. | Ref. | Ref. |
*wynik istotny statystycznie (p<0,05) P.437046
Among men diagnosed with prostate cancer whose serum zinc concentration is in the range of 1000-1200, a nearly 4-fold reduced risk of death was observed compared to men whose zinc concentration is lower than 900 µg/l.
Incidence of death depending on serum zinc concentration in men with prostate cancer within 5 years of diagnosis (selected ranges)
Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
I | <900 | 180 | 54 | 3,7 | 1,09-12,48 | 0,03* |
II | 1000-1200 | 37 | 3 | Ref. | Ref. | Ref. |
*wynik istotny statystycznie (p<0,05) P.437046
The above results are the subject of patent application P.437046.
Survival of patients with laryngeal cancer
Among people with laryngeal cancer, the risk of death was more than twice reduced in patients with zinc concentrations above 688 µg/l in serum compared to those with concentrations <580 µg/l.
Frequency of deaths depending on serum zinc concentration in patients with laryngeal cancer within 5 years of diagnosis (tertiles)
Group | Range [µg/l] | Alive | Deaths | OR | 95%CI | p |
I | 357,76-580,38 | 52 | 52 | 2,45 | 1,4-4,4 | <0,01* |
II | 584,46-688,89 | 67 | 37 | 1,35 | 0,8-2,4 | 0,31 |
III | 688,94-1317,87 | 76 | 31 | Ref. | Ref. | Ref. |
*wynik istotny statystycznie (p<0,05) P.427370
Powyższe wyniki stanowią przedmiot publikacji Lubiński J. et al., Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes, Biomolecules 2021, 11, 865.[40]
Copper performs various functions in the structure of proteins and as a catalyst thanks to the ability to change the degree of oxidation and reduction and occurs in the oxidized (Cu2+) or reduced (Cu+) state. Copper ions can participate in a wide range of interactions with proteins, enabling the formation of complex structures and mediating complex biochemical reactions.[41,42] Copper, like other trace elements, is, on the one hand, essential for the body, but on the other hand, it is very dangerous. . In general, however, conditions that are characterized by general or cell-specific copper accumulation are rare and most often occur as a result of specific genetic disorders.[43]
Evaluation of the cancer risk in women - BRCA1 non-carriers
Non-smoking women under 50 with blood copper concentrations between 850-1000 µg/l have an almost three-fold reduced risk of cancer.
Częstość występowania raków u kobiet niepalących poniżej 50 roku życia, u których nie stwierdzono mutacji w genie BRCA1 (wybrane zakresy).
Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
I | 850-1000 | 16 | 493 | Ref. | Ref. | Ref. |
II | <850 | 13 | 156 | 2,6 | 1,2-5,5 | 0,02* |
*statistically significant result (p<0.05) P.438572
Environmental lead contamination is an ongoing problem for developing societies. The toxic effect of lead mainly concerns its effect on the hematopoietic system[44], the peripheral and central nervous system[45] and the gastrointestinal tract[46]. Due to the ubiquity of lead, virtually every person is exposed to contact with this element. Lead toxicity leads, among other things, to changes in the activity of many enzymes and disturbances in the functions of free and structural proteins in the cell[47]. Many studies suggest that an important molecular mechanism of lead toxicity is its participation in the formation of free oxygen radicals, which play a large role in the formation of intracellular damage and in the pathogenesis of many diseases, including malignant tumors[48]. According to the IARC classification of carcinogens, lead and its compounds belong to groups 2a and 2b, i.e. potentially carcinogenic [12].
Evaluation of the cancer risk in women - BRCA1 non-carriers
Women with blood lead concentration ≤ 8 µg/l have a significantly reduced risk of developing cancer by almost 9 times.
Cancer incidence depends on lead concentration in women. (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | ≤8 | 1 | 372 | Ref. | Ref. | Ref. |
| II | >8 | 26 | 1094 | 8,84 | 1,2-65,41 | 0,006* |
*statistically significant result (p<0.05) P.433150
Evaluation of the cancer risk in women - BRCA1 carriers
Women with blood lead concentration ≤ 8 µg/l have a significantly reduced risk of developing cancer by three times.
Cancer incidence depends on lead concentration in women. (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | ≤8 | 8 | 217 | Ref. | Ref. | Ref. |
| II | >8 | 87 | 812 | 2,9 | 1,4-6,1 | 0,002* |
*statistically significant result (p<0.05) P.433150
Evaluation of the cancer risk in men
Men under 60 years of age and non-smokers have an almost 8-fold reduced risk of developing the disease if their lead concentration is lower than 13.5 µg/l.
Cancer incidence depends on lead concentration in non-smoking men under 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | <13,5 | 1 | 217 | Ref. | Ref. | Ref. |
| II | >13,5 | 16 | 447 | 7,8 | 1,02-59,0 | 0,017* |
*statistically significant result (p<0.05) P.437899
Men over 60 years of age, non-smokers have a four-fold reduced risk of cancer if their lead concentration is in the range of 20-50 µg/l in whole blood.
Cancer incidence depends on lead concentration in non-smoking men over 60 years of age (selected ranges).
| Group | Range [µg/l] | New cancer diagnosis | Unaffected | OR | 95%CI | p |
| I | 20-50 | 3 | 74 | Ref. | Ref. | Ref. |
| II | <20 & >50 | 15 | 90 | 4,1 | 1,1-14,8 | 0,02* |
*statistically significant result (p<0.05) P.437899
Manganese (Mn) is an essential nutrient involved in properly functioning the immune system, regulation of blood sugar levels and cellular energy, reproduction, digestion, bone growth, blood clotting and homeostasis, and defense against reactive oxygen species. The functions performed by manganese metalloproteins include oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. [49] Mn acts as a cofactor for various enzymes, including arginase, glutamine synthetase (GS), pyruvate carboxylase, and Mn superoxide dismutase (Mn-SOD) [50]. Mn tends to accumulate in the liver, pancreas, bones, and brain [50].
The literature contains studies describing the correlation between serum Mn concentration and the risk of breast and colorectal cancer. However, the published studies are retrospective, which makes them unreliable for the conclusion that manganese is a marker of cancer risk. In our Center, we assessed the correlation between manganese concentration and survival of women with breast cancer.
Survival of patients with breast cancer
Women diagnosed with breast cancer, whose serum manganese concentration is in the range of 1-10 µg/l, have a significantly reduced risk of death by over 1.5 times compared to other women.
The incidence of death depends on serum manganese concentration in women with breast cancer within 10 years of diagnosis (selected ranges).
Group | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
I | 1,0-10,0 | 197 | 49 | Ref. | Ref. | Ref. |
II | <1 & > 10 | 204 | 87 | 1,55 | 1,09-2,20 | 0,01* |
*wynik istotny statystycznie (p<0,05) P.438040
Chromium (Cr) is an element widely distributed in the Earth's crust. This element in small amounts is necessary for the proper functioning of the body, playing a role in the metabolic processes of glucose, some proteins, and fats [51–53]. Increased doses of chromium may have a strong toxic effect, destructively affecting the liver, kidneys, and hematopoietic system, and causing cancer [51,54,55]. Cr(VI) has been found to have a strong mutagenic effect. The results of association studies indicate a relationship between the concentration of chromium in the blood and the risk of cancer in the head, neck, and oral cavity.
In our Center, work was carried out on the correlation between serum chromium concentration and survival of women diagnosed with breast cancer.
Survival of patients with breast cancer
Women diagnosed with breast cancer whose serum chromium concentration is in the range of 0.20-3.5 µg/l have a significantly reduced risk of death by almost two times.
Incidence of death depending on serum chromium concentration in women with breast cancer within 10 years of diagnosis (selected ranges).
Group | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
I | 0,20-3,5 | 202 | 54 | Ref. | Ref. | Ref. |
II | <0,20 & > 3,5 | 199 | 82 | 1,8 | 1,28-2,54 | 0,0002* |
*statistically significant result (p<0.05) P.438042
Women who smoke cigarettes and have been diagnosed with breast cancer have a significantly reduced risk of death by almost 4.5 times if their serum chromium concentration is higher than 0.5 µg/l.
Incidence of death depending on serum chromium concentration in smokers with breast cancer within 10 years of diagnosis (selected ranges).
Group | Range [µg/l] | Alive | Deaths | HR | 95%CI | p |
I | <0,5 | 157 | 56 | 4,46 | 1,39-14,26 | 0,01* |
II | ≥0,5 | 38 | 3 | Ref. | Ref. | Ref. |
*statistically significant result (p<0.05) P.438042
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology 2020, 28, 667–695, doi:10.1007/s10787-020-00690-x.
- Combs, G.F.; Clark, L.C.; Turnbull, B.W. An Analysis of Cancer Prevention by Selenium. Biofactors 2001, 14, 153–159, doi:10.1002/biof.5520140120.
- Reddy, V.N.; Giblin, F.J.; Lin, L.R.; Dang, L.; Unakar, N.J.; Musch, D.C.; Boyle, D.L.; Takemoto, L.J.; Ho, Y.S.; Knoernschild, T.; et al. Glutathione Peroxidase-1 Deficiency Leads to Increased Nuclear Light Scattering, Membrane Damage, and Cataract Formation in Gene-Knockout Mice. Invest Ophthalmol Vis Sci 2001, 42, 3247–3255.
- Kuria, A.; Fang, X.; Li, M.; Han, H.; He, J.; Aaseth, J.O.; Cao, Y. Does Dietary Intake of Selenium Protect against Cancer? A Systematic Review and Meta-Analysis of Population-Based Prospective Studies. Crit Rev Food Sci Nutr 2020, 60, 684–694, doi:10.1080/10408398.2018.1548427.
- Jenkins, D.J.A.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Blanco Mejia, S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.C.; et al. Selenium, Antioxidants, Cardiovascular Disease, and All-Cause Mortality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Clin Nutr 2020, 112, 1642–1652, doi:10.1093/ajcn/nqaa245.
- Schomburg, L. The Other View: The Trace Element Selenium as a Micronutrient in Thyroid Disease, Diabetes, and Beyond. Hormones (Athens) 2020, 19, 15–24, doi:10.1007/s42000-019-00150-4.
- Kenfield, S.A.; Van Blarigan, E.L.; DuPre, N.; Stampfer, M.J.; L Giovannucci, E.; Chan, J.M. Selenium Supplementation and Prostate Cancer Mortality. J Natl Cancer Inst 2015, 107, 360, doi:10.1093/jnci/dju360.
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.; Falco, M.; et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients 2021, 13, 953, doi:10.3390/nu13030953.
- Pietrzak, S.; Wójcik, J.; Scott, R.J.; Kashyap, A.; Grodzki, T.; Baszuk, P.; Bielewicz, M.; Marciniak, W.; Wójcik, N.; Dębniak, T.; et al. Influence of the Selenium Level on Overall Survival in Lung Cancer. J Trace Elem Med Biol 2019, 56, 46–51, doi:10.1016/j.jtemb.2019.07.010.
- Lener, M.R.; Scott, R.J.; Wiechowska-Kozłowska, A.; Serrano-Fernández, P.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Marciniak, W.; Muszyńska, M.; Kładny, J.; et al. Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer. Cancer Res Treat 2016, 48, 1056–1064, doi:10.4143/crt.2015.282.
- Rogoża-Janiszewska E. et al Serum Selenium Level and the 10-Year Survival after Melanoma. Biomedicines SI: Role of Trace Elements in Chemoprevention and Cancer Therapy 2021.
- List of Classifications – IARC Monographs on the Identification of Carcinogenic Hazards to Humans Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 20 July 2021).
- Marciniak, W.; Derkacz, R.; Muszyńska, M.; Baszuk, P.; Gronwald, J.; Huzarski, T.; Cybulski, C.; Jakubowska, A.; Falco, M.; Dębniak, T.; et al. Blood Arsenic Levels and the Risk of Familial Breast Cancer in Poland. Int J Cancer 2020, 146, 2721–2727, doi:10.1002/ijc.32595.
- Marciniak, W.; Matoušek, T.; Domchek, S.; Paradiso, A.; Patruno, M.; Irmejs, A.; Roderte, I.; Derkacz, R.; Baszuk, P.; Kuświk, M.; et al. Blood Arsenic Levels as a Marker of Breast Cancer Risk among BRCA1 Carriers. Cancers 2021, 13, 3345, doi:10.3390/cancers13133345.
- Fowler, B.A. Monitoring of Human Populations for Early Markers of Cadmium Toxicity: A Review. Toxicol Appl Pharmacol 2009, 238, 294–300, doi:10.1016/j.taap.2009.05.004.
- Vinceti, M.; Venturelli, M.; Sighinolfi, C.; Trerotoli, P.; Bonvicini, F.; Ferrari, A.; Bianchi, G.; Serio, G.; Bergomi, M.; Vivoli, G. Case-Control Study of Toenail Cadmium and Prostate Cancer Risk in Italy. Sci Total Environ 2007, 373, 77–81, doi:10.1016/j.scitotenv.2006.11.005.
- Qayyum, M.A.; Shah, M.H. Comparative Study of Trace Elements in Blood, Scalp Hair and Nails of Prostate Cancer Patients in Relation to Healthy Donors. Biol Trace Elem Res 2014, 162, 46–57, doi:10.1007/s12011-014-0123-4.
- Pirincci, N.; Gecit, I.; Gunes, M.; Kaba, M.; Tanik, S.; Yuksel, M.B.; Arslan, H.; Demir, H. Levels of Serum Trace Elements in Renal Cell Carcinoma Cases. Asian Pac J Cancer Prev 2013, 14, 499–502, doi:10.7314/apjcp.2013.14.1.499.
- Kellen, E.; Zeegers, M.P.; Hond, E.D.; Buntinx, F. Blood Cadmium May Be Associated with Bladder Carcinogenesis: The Belgian Case-Control Study on Bladder Cancer. Cancer Detect Prev 2007, 31, 77–82, doi:10.1016/j.cdp.2006.12.001.
- Wolf, C.; Strenziok, R.; Kyriakopoulos, A. Elevated Metallothionein-Bound Cadmium Concentrations in Urine from Bladder Carcinoma Patients, Investigated by Size Exclusion Chromatography-Inductively Coupled Plasma Mass Spectrometry. Anal Chim Acta 2009, 631, 218–222, doi:10.1016/j.aca.2008.10.035.
- Farzin, L.; Moassesi, M.E.; Sajadi, F.; Ahmadi Faghih, M.A. Evaluation of Trace Elements in Pancreatic Cancer Patients in Iran. Middle East Journal of Cancer 2013, 4, 79–86.
- Amaral, A.F.S.; Porta, M.; Silverman, D.T.; Milne, R.L.; Kogevinas, M.; Rothman, N.; Cantor, K.P.; Jackson, B.P.; Pumarega, J.A.; López, T.; et al. Pancreatic Cancer Risk and Levels of Trace Elements. Gut 2012, 61, 1583–1588, doi:10.1136/gutjnl-2011-301086.
- Wu, H.-D.I.; Chou, S.-Y.; Chen, D.-R.; Kuo, H.-W. Differentiation of Serum Levels of Trace Elements in Normal and Malignant Breast Patients. Biol Trace Elem Res 2006, 113, 9–18, doi:10.1385/BTER:113:1:19.
- Deeb, M.; El-Sheredy, H.; Mohammed, A. The Role of Serum Trace Elements and Oxidative Stress in Egyptian Breast Cancer Patients. Advances in Breast Cancer Research 2016, 05, 37–47, doi:10.4236/abcr.2016.51004.
- Sabir, S.; Akash, M.S.H.; Fiayyaz, F.; Saleem, U.; Mehmood, M.H.; Rehman, K. Role of Cadmium and Arsenic as Endocrine Disruptors in the Metabolism of Carbohydrates: Inserting the Association into Perspectives. Biomed Pharmacother 2019, 114, 108802, doi:10.1016/j.biopha.2019.108802.
- Lee, J.-E.; Kim, H.-R.; Lee, M.; Kim, N.-H.; Wang, K.-M.; Lee, S.; Park, O.; Hong, E.-J.; Youn, J.-W.; Kim, Y.-Y. Smoking-Related DNA Methylation Is Differentially Associated with Cadmium Concentration in Blood. Biochem Genet 2020, 58, 617–630, doi:10.1007/s10528-020-09965-y.
- Bolam, T.; Bersuder, P.; Burden, R.; Shears, G.; Morris, S.; Warford, L.; Thomas, B.; Nelson, P. Cadmium Levels in Food Containing Crab Brown Meat: A Brief Survey from UK Retailers. Journal of Food Composition and Analysis 2016, 54, 63–69, doi:10.1016/j.jfca.2016.10.005.
- Derkacz R. et al. Blood Cadmium Level and the Risk of Cancer in Women with BRCA1 Mutations. Cancers, SI: Advances in Inherited Breast and Ovarian Cancer and Its Imaging 2021.
- Nasiadek, M.; Stragierowicz, J.; Klimczak, M.; Kilanowicz, A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients 2020, 12, 2464, doi:10.3390/nu12082464.
- Puzanowska-Tarasiewicz, H.; Kuźmicka, L.; Tarasiewicz, M. Funkcje biologiczne wybranych pierwiastków i ich związków. 3, 3,. Polski Merkuriusz Lekarski : organ Polskiego Towarzystwa Lekarskiego. 2009, 419–422.
- To, P.K.; Do, M.H.; Cho, J.-H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int J Mol Sci 2020, 21, E2991, doi:10.3390/ijms21082991.
- Zaichick VYe, null; Sviridova, T.V.; Zaichick, S.V. Zinc in the Human Prostate Gland: Normal, Hyperplastic and Cancerous. Int Urol Nephrol 1997, 29, 565–574, doi:10.1007/BF02552202.
- Siddiqui, M.K.J.; Jyoti, null; Singh, S.; Mehrotra, P.K.; Singh, K.; Sarangi, R. Comparison of Some Trace Elements Concentration in Blood, Tumor Free Breast and Tumor Tissues of Women with Benign and Malignant Breast Lesions: An Indian Study. Environ Int 2006, 32, 630–637, doi:10.1016/j.envint.2006.02.002.
- Pasha, Q.; Malik, S.A.; Shah, M.H. Statistical Analysis of Trace Metals in the Plasma of Cancer Patients versus Controls. J Hazard Mater 2008, 153, 1215–1221, doi:10.1016/j.jhazmat.2007.09.115.
- el-Ahmady, O.; el-Maraghy, A.; Ibrahim, A.; Ramzy, S. Serum Copper, Zinc, and Iron in Patients with Malignant and Benign Pulmonary Diseases. Nutrition 1995, 11, 498–501.
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol Trace Elem Res 2002, 89, 1–11, doi:10.1385/BTER:89:1:1.
- Zhou, W.; Park, S.; Liu, G.; Miller, D.P.; Wang, L.I.; Pothier, L.; Wain, J.C.; Lynch, T.J.; Giovannucci, E.; Christiani, D.C. Dietary Iron, Zinc, and Calcium and the Risk of Lung Cancer. Epidemiology 2005, 16, 772–779, doi:10.1097/01.ede.0000181311.11585.59.
- Zhang, X.; Giovannucci, E.L.; Smith-Warner, S.A.; Wu, K.; Fuchs, C.S.; Pollak, M.; Willett, W.C.; Ma, J. A Prospective Study of Intakes of Zinc and Heme Iron and Colorectal Cancer Risk in Men and Women. Cancer Causes Control 2011, 22, 1627–1637, doi:10.1007/s10552-011-9839-z.
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc Supplement Use and Risk of Prostate Cancer. J Natl Cancer Inst 2003, 95, 1004–1007, doi:10.1093/jnci/95.13.1004.
- Lubiński, J.; Jaworowska, E.; Derkacz, R.; Marciniak, W.; Białkowska, K.; Baszuk, P.; Scott, R.J.; Lubiński, J.A. Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes. Biomolecules 2021, 11, 865, doi:10.3390/biom11060865.
- Festa, R.A.; Thiele, D.J. Copper: An Essential Metal in Biology. Curr Biol 2011, 21, R877-883, doi:10.1016/j.cub.2011.09.040.
- Ceramella, J.; Mariconda, A.; Iacopetta, D.; Saturnino, C.; Barbarossa, A.; Caruso, A.; Rosano, C.; Sinicropi, M.S.; Longo, P. From Coins to Cancer Therapy: Gold, Silver and Copper Complexes Targeting Human Topoisomerases. Bioorg Med Chem Lett 2020, 30, 126905, doi:10.1016/j.bmcl.2019.126905.
- Linder, M.C. The Relationship of Copper to DNA Damage and Damage Prevention in Humans. Mutat Res 2012, 733, 83–91, doi:10.1016/j.mrfmmm.2012.03.010.
- Johnson, F.M. The Genetic Effects of Environmental Lead. Mutat Res 1998, 410, 123–140, doi:10.1016/s1383-5742(97)00032-x.
- Marchetti, C. Molecular Targets of Lead in Brain Neurotoxicity. Neurotox Res 2003, 5, 221–236, doi:10.1007/BF03033142.
- Tomczyk, J.; Lewczuk, E.; Abdrzejak, R. Ostre Zatrucia Organicznymi Związkammi Ołowiu.; Medycyna Pracy, 1999; Vol. 50;.
- Cellular Mechanisms of Lead Neurotoxicity – PubMed Available online: https://pubmed.ncbi.nlm.nih.gov/16501435/ (accessed on 20 July 2021).
- Nersesyan, A.; Kundi, M.; Waldherr, M.; Setayesh, T.; Mišík, M.; Wultsch, G.; Filipic, M.; Mazzaron Barcelos, G.R.; Knasmueller, S. Results of Micronucleus Assays with Individuals Who Are Occupationally and Environmentally Exposed to Mercury, Lead and Cadmium. Mutat Res 2016, 770, 119–139, doi:10.1016/j.mrrev.2016.04.002.
- Aschner, M.; Erikson, K. Manganese. Adv Nutr 2017, 8, 520–521, doi:10.3945/an.117.015305.
- Chen, P. Manganese Metabolism in Humans. Front Biosci 2018, 23, 1655–1679, doi:10.2741/4665.
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and Oxidative Mechanisms of Different Forms of Chromium. Toxicology 2002, 180, 5–22, doi:10.1016/s0300-483x(02)00378-5.
- Cefalu, W.T.; Hu, F.B. Role of Chromium in Human Health and in Diabetes. Diabetes Care 2004, 27, 2741–2751, doi:10.2337/diacare.27.11.2741.
- Vincent, J.B. The Biochemistry of Chromium. J Nutr 2000, 130, 715–718, doi:10.1093/jn/130.4.715.
- Anderson, R.A. Chromium Metabolism and Its Role in Disease Processes in Man. Clin Physiol Biochem 1986, 4, 31–41.
- Anderson, R.A. Chromium as an Essential Nutrient for Humans. Regul Toxicol Pharmacol 1997, 26, S35-41, doi:10.1006/rtph.1997.1136.
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum Selenium Levels Predict Survival after Breast Cancer. Breast Cancer Res Treat 2018, 167, 591–598, doi:10.1007/s10549-017-4525-9.
- Lubiński, J.; Marciniak, W.; Muszynska, M.; Jaworowska, E.; Sulikowski, M.; Jakubowska, A.; Kaczmarek, K.; Sukiennicki, G.; Falco, M.; Baszuk, P.; et al. Serum Selenium Levels and the Risk of Progression of Laryngeal Cancer. PLoS One 2018, 13, e0184873, doi:10.1371/journal.pone.0184873.
